COVID-19-associated coagulopathy: Difference between revisions
Ifrah Fatima (talk | contribs) |
No edit summary |
||
| Line 30: | Line 30: | ||
==Pathophysiology== | ==Pathophysiology== | ||
[[COVID-19]] induces a [[Hypercoagulable state|hypercoagulable]] state in the body. An increased risk of [[mortality]] has been noted in patient’s with [[coagulopathy]] in COVID-19. <ref name="pmid32073213">{{cite journal| author=Tang N, Li D, Wang X, Sun Z| title=Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. | journal=J Thromb Haemost | year= 2020 | volume= 18 | issue= 4 | pages= 844-847 | pmid=32073213 | doi=10.1111/jth.14768 | pmc=7166509 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32073213 }} </ref> | * [[COVID-19]] induces a [[Hypercoagulable state|hypercoagulable]] state in the body. An increased risk of [[mortality]] has been noted in patient’s with [[coagulopathy]] in COVID-19. <ref name="pmid32073213">{{cite journal| author=Tang N, Li D, Wang X, Sun Z| title=Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. | journal=J Thromb Haemost | year= 2020 | volume= 18 | issue= 4 | pages= 844-847 | pmid=32073213 | doi=10.1111/jth.14768 | pmc=7166509 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32073213 }} </ref> It is thought that the [[coagulopathy]] in COVID-19 is the result of:<ref name="pmid32415579" /> | ||
It is thought that the [[coagulopathy]] in COVID-19 is the result of | ** [[Virchow's triad|Virchow]]’s triad | ||
*[[Virchow's triad|Virchow]]’s triad | ** Vascular [[Endothelium|endothelial]] damage | ||
**Vascular [[Endothelium|endothelial]] damage | |||
**Endothelitis- direct invasion of endothelial cells by SARS-CoV-2 | **Endothelitis- direct invasion of endothelial cells by SARS-CoV-2 | ||
**[[Complement system|Complement]] mediated damage to pericytes | **[[Complement system|Complement]] mediated damage to pericytes | ||
| Line 43: | Line 42: | ||
==Causes== | ==Causes== | ||
[[Coagulopathy]] in COVID-19 is caused by- | [[Coagulopathy]] in COVID-19 is caused by:<ref name="LiLiu2020">{{cite journal|last1=Li|first1=Zhengqian|last2=Liu|first2=Taotao|last3=Yang|first3=Ning|last4=Han|first4=Dengyang|last5=Mi|first5=Xinning|last6=Li|first6=Yue|last7=Liu|first7=Kaixi|last8=Vuylsteke|first8=Alain|last9=Xiang|first9=Hongbing|last10=Guo|first10=Xiangyang|title=Neurological manifestations of patients with COVID-19: potential routes of SARS-CoV-2 neuroinvasion from the periphery to the brain|journal=Frontiers of Medicine|year=2020|issn=2095-0217|doi=10.1007/s11684-020-0786-5}}</ref> | ||
* Direct invasion of endothelial cells by SARS-CoV-2 | * Direct invasion of endothelial cells by SARS-CoV-2 | ||
* Pro-inflammatory cytokine storm. | * Pro-inflammatory cytokine storm. | ||
Revision as of 10:41, 27 June 2020
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ifrah Fatima, M.B.B.S[2]
Synonyms and keywords:
Overview
Historical Perspective
- Coronavirus, named due to the "crown" like appearance of its surface projections, was first isolated from chickens in 1937.,
- In 1965, Tyrrell and Bynoe used cultures of human ciliated embryonal trachea to propagate the first human coronavirus (HCoV) in vitro.
- There are now approximately 15 species in this family, which infect not only humans but cattle, pigs, rodents, cats, dogs and birds (some are serious veterinary pathogens, especially chickens).[1]
- The etiological agent, a novel coronavirus, SARS-CoV-2, named for the similarity of its symptoms to those induced by the severe acute respiratory syndrome, causing coronavirus disease 2019 (COVID-19), is a virus identified as the cause of an outbreak of respiratory illness first detected in Wuhan, China.[2][3]
- Initially, the patients were believed to have contracted the virus from seafood/animal markets which suggested animal-to-human spread.
- The growing number of patients however, suggest that human-to-human transmission is actively occurring.[4][5]
- The outbreak was declared a Public Health Emergency of International Concern on 30 January 2020.
- On March 12, 2020 the World Health Organization declared the COVID-19 outbreak a pandemic.
Classification
There is no established system for the classification of the hypercoagulability seen in COVID-19.
The coagulopathy may be classified according to the type of vessels and organs involved into:[6]
- Venous thrombosis
- Arterial thrombosis
- Microvascular thrombosis
Pathophysiology
- COVID-19 induces a hypercoagulable state in the body. An increased risk of mortality has been noted in patient’s with coagulopathy in COVID-19. [7] It is thought that the coagulopathy in COVID-19 is the result of:[6]
- Virchow’s triad
- Vascular endothelial damage
- Endothelitis- direct invasion of endothelial cells by SARS-CoV-2
- Complement mediated damage to pericytes
- Pro-inflammatory cytokines- IL-1, IL-6, and TNF- α, that activate the coagulation pathway and the fibrinolytic system
- Stasis- Prolonged hospital admissions causing immobilization of the patient
- Hypercoagulabe state- Evidenced by elevated fibrinogen, prothrombotic factors and hyperviscosity [8]
- Some patients have been found to have Lupus anticoagulant (anti-cardiolipin) and anti-β2GP1 antibodies that may be contributory. [9]
Causes
Coagulopathy in COVID-19 is caused by:[10]
- Direct invasion of endothelial cells by SARS-CoV-2
- Pro-inflammatory cytokine storm.
- Prolonged immobilization in hospitalized patients causing stasis.
Differentiating COVID-19 associated coagulopathy from other Diseases
Coagulopathy in COVID-19 must be differentiated from other diseases that cause DIC.
The main feature of COVID-19 coagulopathy is thrombosis while the acute phase of DIC presents with bleeding: [11]
- Similar laboratory findings are marked increase in D-dimer and normal/slightly low platelets and prolonged PT.
- Findings distinct in COVID 19 are high fibrinogen and high factor VIII activity
- The scoring system of the International Society on Thrombosis and Hemostasis should be used to detect DIC (platelet count, PT, fibrinogen, D‐dimer, antithrombin and protein C activity monitoring), but the diagnosis and subsequent treatment should be done clinically. [12]
Epidemiology and Demographics
The incidence of venous thromboembolism in ICU patients with COVID-19 was analyzed in a study by Klok et al. [13]
- It concluded that the cumulative incidence of acute pulmonary embolism (PE), deep vein thrombosis (DVT), ischemic stroke, MI, or systemic arterial embolism was 31%.
- The most common thrombotic complication was pulmonary embolism seen in 81% of patients. All these patients were on at least standard doses of thromboprophylaxis. [13]
- The cumulative incidences of VTE were 16% (95% CI, 10-22) at 7 days, 33% (95% CI, 23-43) at 14 days and 42% (95% CI 30-54) at 21 days.
- Comparatively, the cumulative incidence of VTE was higher in the ICU patients - 26% (95% CI, 17-37) at 7 days, 47% (95% CI, 34-58) at 14 days and 59% (95% CI, 42-72) at 21 days) than on the floor. [14]
Independent predictors of thrombotic complications seen were-
- Age
- Coagulopathy (defined as spontaneous prolongation of the prothrombin time > 3 s or activated partial thromboplastin time > 5 s)
- Active cancer
There is no data on gender, geographic location, and racial predilection to coagulopathy in COVID-19.
Risk Factors
Hypothesized risk factors for coagulopathy in COVID-19 pneumonia based on studies include-
- ICU admission [13]
- Age (> 40 years)
- Hypoxia
Other general risk factors for VTE are-
- Male
- Active cancer
- Recent major surgery
- High BMI (obesity)
- Prior VTE
- Immobilization
- Trauma
Screening
Every patient with COVID-19 infection admitted to the hospital should have a baseline of basic blood investigations such as
- Complete blood count (CBC)
- Platelet count
- Prothrombin Time (PT), Activated partial thromboplastin time (aPTT)
- Fibrinogen
- D-dimer
- C- reactive protein
Routine screening with imaging is not done as there is not evidence to indicate an improvement in clinical outcomes. Depending on the clinical state of the patient and suspicion for the development of VTE or arterial thrombi, repeat testing and further imaging investigations are done.
Natural History, Complications, and Prognosis
Natural History
If left untreated, patients with coagulopathy may progress to develop VTE, arterial thrombosis, or microvascular thrombosis and ultimately succumb to death.
Complications
- Thrombotic complications : [6] [15]
- Deep Vein Thrombosis
- Pulmonary Embolism
- Ischemic stroke
- Myocardial infarction
- Ischemic limbs
- Systemic arterial events
- Clotting of central venous catheters, dialysis catheters, and dialysis filters
Prognosis
Prognosis depends on numerous factors-
- Increased D-dimer levels- poor prognosis [16]
- Increased fibrin degradation product (FDP) levels [7]
- ICU admission
Diagnosis
Diagnostic Study of Choice
- The diagnosis of coagulopathy in COVID-19 is based mainly on the laboratory findings showing a pro-coagulant profile.
- The pre-test probability of DVT and PE can be calculated using the Wells' criteria
- Computed Tomography with pulmonary angiography (CTPA) is the diagnostic test of choice. Ventilation/Perfusion scan may also be done, but may not be of much yield in patients with COVID-19.
History and Symptoms
The symptoms depend on the vessels and the organ systems involved.
Pulmonary Embolism- Many symptoms of PE overlap with the respiratory symptoms seen in COVID-19.
- Maybe asymptomatic
- Dyspnea
- Chest pain
- Cough
- Some other rare presentations include- hemoptysis, shock, hypotension, death.
A positive history of the following is suggestive of and contributory-
- Immobilization or prolonged hospitalization
- Recent surgery
- Trauma
- Obesity
- History of previous venous thromboembolism (VTE)
- Malignancy
- Stroke with hemiplegia or immobility
- Age >65 years
Deep Vein Thrombosis-
Arterial thrombosis involving various systems show the following symptoms-
- Ischemic Stroke- various focal neurological deficits depending on the large artery involved
- Myocardial infarction- Chest pain radiating to left arm and neck, sweating, dyspnea
- Acute ischemic limb- pain, pallor, poikilothermia, pulselessness, paresthesia, paralysis
Physical Examination
Pulmonary Embolism Physical examination of patients with [Pulmonary Embolism] is usually remarkable for-
- Tachycardia
- Tachypnea
- Diaphoresis
Deep Vein Thrombosis Physical examination of patients with Deep Vein Thrombosis includes-
- Unilateral swelling/edema with a difference in diameters
- Warmth
- Tenderness
- Dilated veins
- Homan's sign may also be seen but is unreliable.
Arterial thrombosis-
- Ischemic Stroke- Focal neurological deficits depending on the vessel involved
- Myocardial Infarction- uncomfortable appearing patient with diaphoresis
- Ischemic Limb- pallor, poikilothermia, pulselessness, paresthesia, paralysis
Laboratory Findings
An elevated concentration of serum/blood pro-coagulant factors is diagnostic of coagulopathy associated with COVID-19. Laboratory findings consistent with the diagnosis of COVID-19 associated coagulopathy include-
Coagulation testing- Pro-coagulant profile [17]
- Platelet Counts- Normal or increased
- Prothrombin time (PT) and activated partial thromboplastin time (aPTT)- normal or slightly prolonged
- Fibrinogen- increased
- D-dimer- increased
- Factor VIII activity- increased
- VWF antigen- increased
- Protein C, Protein S, Antithrombin III - slightly decreased
- Reaction time (R) - decreased
- Clot formation time (K)- decreased
- Maximum amplitude (MA)- increased
- Clot lysis at 30 minutes (LY30)- decreased
Electrocardiogram
An ECG may be helpful in the diagnosis of pulmonary embolism or myocardial infacrctioncaused due to hypercoagulability in COVID-19.
- Findings on an ECG suggestive of/diagnostic of pulmonary embolism include tachycardia and S1Q3T3 pattern.
- Findings on an ECG suggestive of/diagnostic of myocardial infarction include STE elevation in various leads.
X-ray
There are no specific x-ray findings associated with PE. However, an x-ray may be helpful in ruling out other causes with similar symptoms like pneumonia, cardiogenic causes of dyspnea, and pneumothorax.
Echocardiography or Ultrasound
- Echocardiography may be helpful in the diagnosis of pulmonary embolism.
- Compressive Ultrasound may be helpful in the diagnosis of deep vein thrombosis
CT scan
CTPA and Ventilation Perfusion(V/Q) Scan
- Prompt diagnosis of PE in COVID-19 patient is difficult in this regard that various symptoms of COVID-19 overlap with that of pulmonary embolism. American Society of Hematology provides the following guidelines regarding the diagnosis of pulmonary embolism:[19]
- Normal d-dimers level in a patient with low to moderate pretest probability is sufficient to rule out the diagnosis of PE. D-dimers level is usually elevated in COVID-19 patients. This is not applicable to a patient with a high pretest probability.
- Inpatient with suspected PE with symptoms like hypotension, tachycardia, and sudden drop in oxygen saturation with a high pretest probability of PE, computed tomography with pulmonary angiography is used for the diagnosis. Contraindication to the use of CTPA warrants investigation with ventilation/perfusion scan.
MRI
There are no MRI findings associated with coagulopathy of COVID-19 unless it is used to diagnose and evaluate an ischemic stroke caused by it.
Other Imaging Findings
There are no other imaging findings associated with coagulopathy of COVID-19
Other Diagnostic Studies
There are no other diagnostic studies associated with any of the manifestations of COVID-19 coagulopathy.
Treatment
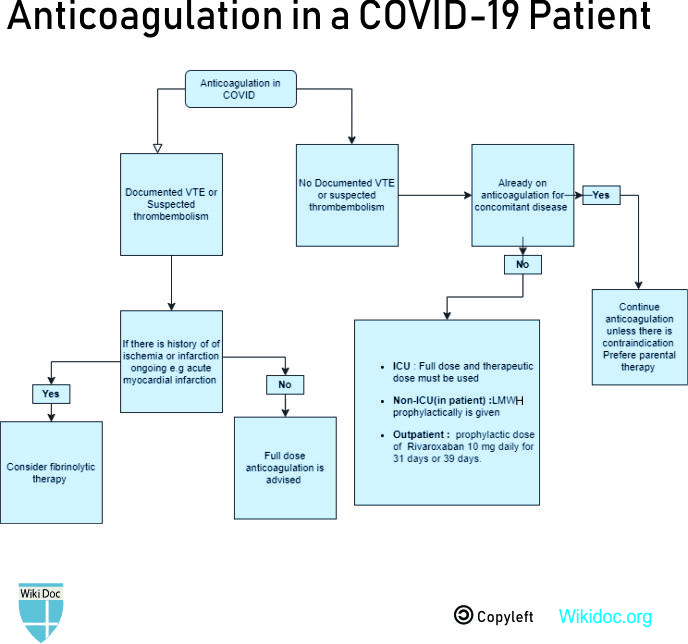
Medical Therapy
Prophylactic
References
- ↑ https://www.cdc.gov/coronavirus/2019-ncov/about/index.html. Missing or empty
|title=(help) - ↑ Lu, Jian; Cui, Jie; Qian, Zhaohui; Wang, Yirong; Zhang, Hong; Duan, Yuange; Wu, Xinkai; Yao, Xinmin; Song, Yuhe; Li, Xiang; Wu, Changcheng; Tang, Xiaolu (2020). "On the origin and continuing evolution of SARS-CoV-2". National Science Review. doi:10.1093/nsr/nwaa036. ISSN 2095-5138.
- ↑ Huang, Chaolin; Wang, Yeming; Li, Xingwang; Ren, Lili; Zhao, Jianping; Hu, Yi; Zhang, Li; Fan, Guohui; Xu, Jiuyang; Gu, Xiaoying; Cheng, Zhenshun; Yu, Ting; Xia, Jiaan; Wei, Yuan; Wu, Wenjuan; Xie, Xuelei; Yin, Wen; Li, Hui; Liu, Min; Xiao, Yan; Gao, Hong; Guo, Li; Xie, Jungang; Wang, Guangfa; Jiang, Rongmeng; Gao, Zhancheng; Jin, Qi; Wang, Jianwei; Cao, Bin (2020). "Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China". The Lancet. 395 (10223): 497–506. doi:10.1016/S0140-6736(20)30183-5. ISSN 0140-6736.
- ↑ https://www.cdc.gov/coronavirus/2019-ncov/about/transmission.html. Missing or empty
|title=(help) - ↑ 6.0 6.1 6.2 Becker RC (2020). "COVID-19 update: Covid-19-associated coagulopathy". J Thromb Thrombolysis. 50 (1): 54–67. doi:10.1007/s11239-020-02134-3. PMC 7225095 Check
|pmc=value (help). PMID 32415579 Check|pmid=value (help). - ↑ 7.0 7.1 Tang N, Li D, Wang X, Sun Z (2020). "Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia". J Thromb Haemost. 18 (4): 844–847. doi:10.1111/jth.14768. PMC 7166509 Check
|pmc=value (help). PMID 32073213 Check|pmid=value (help). - ↑ Maier CL, Truong AD, Auld SC, Polly DM, Tanksley CL, Duncan A (2020). "COVID-19-associated hyperviscosity: a link between inflammation and thrombophilia?". Lancet. 395 (10239): 1758–1759. doi:10.1016/S0140-6736(20)31209-5. PMC 7247793 Check
|pmc=value (help). PMID 32464112 Check|pmid=value (help). - ↑ Bowles L, Platton S, Yartey N, Dave M, Lee K, Hart DP; et al. (2020). "Lupus Anticoagulant and Abnormal Coagulation Tests in Patients with Covid-19". N Engl J Med. doi:10.1056/NEJMc2013656. PMC 7217555 Check
|pmc=value (help). PMID 32369280 Check|pmid=value (help). - ↑ Li, Zhengqian; Liu, Taotao; Yang, Ning; Han, Dengyang; Mi, Xinning; Li, Yue; Liu, Kaixi; Vuylsteke, Alain; Xiang, Hongbing; Guo, Xiangyang (2020). "Neurological manifestations of patients with COVID-19: potential routes of SARS-CoV-2 neuroinvasion from the periphery to the brain". Frontiers of Medicine. doi:10.1007/s11684-020-0786-5. ISSN 2095-0217.
- ↑ Levi M, Thachil J, Iba T, Levy JH (2020). "Coagulation abnormalities and thrombosis in patients with COVID-19". Lancet Haematol. 7 (6): e438–e440. doi:10.1016/S2352-3026(20)30145-9. PMC 7213964 Check
|pmc=value (help). PMID 32407672 Check|pmid=value (help). - ↑ Levi M, Toh CH, Thachil J, Watson HG (2009). "Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology". Br J Haematol. 145 (1): 24–33. doi:10.1111/j.1365-2141.2009.07600.x. PMID 19222477.
- ↑ 13.0 13.1 13.2 Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM; et al. (2020). "Incidence of thrombotic complications in critically ill ICU patients with COVID-19". Thromb Res. 191: 145–147. doi:10.1016/j.thromres.2020.04.013. PMC 7146714 Check
|pmc=value (help). PMID 32291094 Check|pmid=value (help). - ↑ Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Müller MCA; et al. (2020). "Incidence of venous thromboembolism in hospitalized patients with COVID-19". J Thromb Haemost. doi:10.1111/jth.14888. PMID 32369666 Check
|pmid=value (help). - ↑ Barrett CD, Moore HB, Yaffe MB, Moore EE (2020). "ISTH interim guidance on recognition and management of coagulopathy in COVID-19: A comment". J Thromb Haemost. doi:10.1111/jth.14860. PMID 32302462 Check
|pmid=value (help). - ↑ Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z; et al. (2020). "D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19". J Thromb Haemost. 18 (6): 1324–1329. doi:10.1111/jth.14859. PMC 7264730 Check
|pmc=value (help). PMID 32306492 Check|pmid=value (help). - ↑ Ranucci M, Ballotta A, Di Dedda U, Bayshnikova E, Dei Poli M, Resta M; et al. (2020). "The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome". J Thromb Haemost. doi:10.1111/jth.14854. PMID 32302448 Check
|pmid=value (help). - ↑ Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V; et al. (2020). "Hypercoagulability of COVID-19 patients in Intensive Care Unit. A Report of Thromboelastography Findings and other Parameters of Hemostasis". J Thromb Haemost. doi:10.1111/jth.14850. PMID 32302438 Check
|pmid=value (help). - ↑ "COVID-19 and Pulmonary Embolism". Hematology.org. 2020-05-18. Retrieved 2020-06-23.