Bortezomib: Difference between revisions
No edit summary |
No edit summary |
||
| Line 66: | Line 66: | ||
'''Stability''': Unopened vials of VELCADE are stable until the date indicated on the package when stored in the original package protected from light. | '''Stability''': Unopened vials of VELCADE are stable until the date indicated on the package when stored in the original package protected from light. | ||
VELCADE contains no antimicrobial preservative. Reconstituted VELCADE should be administered within 8 hours of preparation. When reconstituted as directed, VELCADE may be stored at 25°C (77°F). The reconstituted material may be stored in the original vial and/or the syringe prior to administration. The product may be stored for up to 8 hours in a syringe; however, total storage time for the reconstituted material must not exceed 8 hours when exposed to normal indoor lighting. | VELCADE contains no antimicrobial preservative. Reconstituted VELCADE should be administered within 8 hours of preparation. When reconstituted as directed, VELCADE may be stored at 25°C (77°F). The reconstituted material may be stored in the original vial and/or the syringe prior to administration. The product may be stored for up to 8 hours in a syringe; however, total storage time for the reconstituted material must not exceed 8 hours when exposed to normal indoor lighting. | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Bortezomib in adult patients. | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Bortezomib in adult patients. | ||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Bortezomib in adult patients. | |offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Bortezomib in adult patients. | ||
| Line 118: | Line 116: | ||
====Embryo-fetal Risk==== | ====Embryo-fetal Risk==== | ||
Women of reproductive potential should avoid becoming pregnant while being treated with VELCADE. Bortezomib administered to rabbits during organogenesis at a dose approximately 0.5 times the clinical dose of 1.3 mg/m2 based on body surface area caused post-implantation loss and a decreased number of live fetuses | Women of reproductive potential should avoid becoming pregnant while being treated with VELCADE. Bortezomib administered to rabbits during organogenesis at a dose approximately 0.5 times the clinical dose of 1.3 mg/m2 based on body surface area caused post-implantation loss and a decreased number of live fetuses | ||
|clinicalTrials=Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice. | |clinicalTrials=Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice. | ||
| Line 266: | Line 264: | ||
‘’‘Skin and subcutaneous tissue disorders’‘’: Urticaria, face edema, rash (which may be pruritic), leukocytoclastic vasculitis, pruritus. | ‘’‘Skin and subcutaneous tissue disorders’‘’: Urticaria, face edema, rash (which may be pruritic), leukocytoclastic vasculitis, pruritus. | ||
‘’‘Vascular disorders’‘’: Cerebrovascular accident, cerebral hemorrhage, deep venous thrombosis, hypertension, peripheral embolism, pulmonary embolism, pulmonary hypertension | ‘’‘Vascular disorders’‘’: Cerebrovascular accident, cerebral hemorrhage, deep venous thrombosis, hypertension, peripheral embolism, pulmonary embolism, pulmonary hypertension | ||
|postmarketing=The following adverse reactions have been identified from the worldwide postmarketing experience with VELCADE. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure: atrioventricular block complete, cardiac tamponade, ischemic colitis, encephalopathy, dysautonomia, deafness bilateral, disseminated intravascular coagulation, hepatitis, acute pancreatitis, progressive multifocal leukoencephalopathy (PML), acute diffuse infiltrative pulmonary disease, PRES (formerly RPLS), toxic epidermal necrolysis, acute febrile neutrophilic dermatosis (Sweet's syndrome), herpes meningoencephalitis, optic neuropathy, blindness and ophthalmic herpes. | |||
|drugInteractions=Bortezomib is a substrate of cytochrome P450 enzyme 3A4, 2C19 and 1A2. | |||
====CYP3A4 inhibitors==== | |||
Co-administration of ketoconazole, a strong CYP3A4 inhibitor, increased the exposure of bortezomib by 35% in 12 patients. Monitor patients for signs of bortezomib toxicity and consider a bortezomib dose reduction if bortezomib must be given in combination with strong CYP3A4 inhibitors (e.g. ketoconazole, ritonavir). | |||
====CYP2C19 inhibitors==== | |||
Co-administration of omeprazole, a strong inhibitor of CYP2C19, had no effect on the exposure of bortezomib in 17 patients. | |||
====CYP3A4 inducers==== | |||
Co-administration of rifampin, a strong CYP3A4 inducer, is expected to decrease the exposure of bortezomib by at least 45%. Because the drug interaction study (n=6) was not designed to exert the maximum effect of rifampin on bortezomib PK, decreases greater than 45% may occur. | |||
Efficacy may be reduced when VELCADE is used in combination with strong CYP3A4 inducers; therefore, concomitant use of strong CYP3A4 inducers is not recommended in patients receiving VELCADE. | |||
St. John's Wort (Hypericum perforatum) may decrease bortezomib exposure unpredictably and should be avoided. | |||
====Dexamethasone==== | |||
Co-administration of dexamethasone, a weak CYP3A4 inducer, had no effect on the exposure of bortezomib in 7 patients. | |||
====Melphalan-Prednisone==== | |||
Co-administration of melphalan-prednisone increased the exposure of bortezomib by 17% in 21 patients. However, this increase is unlikely to be clinically relevant. | |||
|FDAPregCat=D | |||
|useInPregnancyFDA=Bortezomib was not teratogenic in nonclinical developmental toxicity studies in rats and rabbits at the highest dose tested (0.075 mg/kg; 0.5 mg/m2 in the rat and 0.05 mg/kg; 0.6 mg/m2 in the rabbit) when administered during organogenesis. These dosages are approximately half the clinical dose of 1.3 mg/m2 based on body surface area. | |||
Pregnant rabbits given bortezomib during organogenesis at a dose of 0.05mg/kg (0.6 mg/m2) experienced significant post-implantation loss and decreased number of live fetuses. Live fetuses from these litters also showed significant decreases in fetal weight. The dose is approximately 0.5 times the clinical dose of 1.3 mg/m2 based on body surface area. | |||
There are no adequate and well-controlled studies in pregnant women. If VELCADE is used during pregnancy, or if the patient becomes pregnant while receiving this drug, the patient should be apprised of the potential hazard to the fetus. | |||
|useInNursing=It is not known whether bortezomib is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from VELCADE, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. | |||
|useInPed=The safety and effectiveness of VELCADE in children have not been established. | |||
|useInGeri=Of the 669 patients enrolled in the relapsed multiple myeloma study, 245 (37%) were 65 years of age or older: 125 (38%) on the VELCADE arm and 120 (36%) on the dexamethasone arm. Median time to progression and median duration of response for patients ≥ 65 were longer on VELCADE compared to dexamethasone [5.5 mo versus 4.3 mo, and 8.0 mo versus 4.9 mo, respectively]. On the VELCADE arm, 40% (n=46) of evaluable patients aged ≥ 65 experienced response (CR+PR) versus 18% (n=21) on the dexamethasone arm. The incidence of Grade 3 and 4 events was 64%, 78% and 75% for VELCADE patients ≤ 50, 51-64 and ≥ 65 years old, respectively. | |||
No overall differences in safety or effectiveness were observed between patients ≥ age 65 and younger patients receiving VELCADE; but greater sensitivity of some older individuals cannot be ruled out. | |||
|useInRenalImpair=The exposure of bortezomib is increased in patients with moderate (bilirubin ≥ 1.5 – 3× ULN) and severe (bilirubin > 3 × ULN) hepatic impairment. Starting dose should be reduced in those patients | |||
|useInHepaticImpair=The exposure of bortezomib is increased in patients with moderate (bilirubin ≥ 1.5 – 3× ULN) and severe (bilirubin > 3 × ULN) hepatic impairment. Starting dose should be reduced in those patients | |||
|othersTitle=Patients with Diabetes | |||
|useInOthers=During clinical trials, hypoglycemia and hyperglycemia were reported in diabetic patients receiving oral hypoglycemics. Patients on oral anti-diabetic agents receiving VELCADE treatment may require close monitoring of their blood glucose levels and adjustment of the dose of their anti-diabetic medication. | |||
|administration=intravenous injection or subcutaneous | |||
|monitoring=FDA Package Insert for Bortezomib contains no information regarding drug monitoring. | |||
|IVCompat=There is limited information about the IV Compatibility. | |||
|overdose=There is no known specific antidote for VELCADE overdosage. In humans, fatal outcomes following the administration of more than twice the recommended therapeutic dose have been reported, which were associated with the acute onset of symptomatic hypotension (5.2) and thrombocytopenia (5.7). In the event of an overdosage, the patient's vital signs should be monitored and appropriate supportive care given. | |||
Studies in monkeys and dogs showed that intravenous bortezomib doses as low as 2 times the recommended clinical dose on a mg/m2 basis were associated with increases in heart rate, decreases in contractility, hypotension, and death. In dog studies, a slight increase in the corrected QT interval was observed at doses resulting in death. In monkeys, doses of 3.0 mg/m2 and greater (approximately twice the recommended clinical dose) resulted in hypotension starting at 1 hour post-administration, with progression to death in 12 to 14 hours following drug administration. | |||
|drugBox={{Drugbox2 | |||
| verifiedrevid = 459493462 | |||
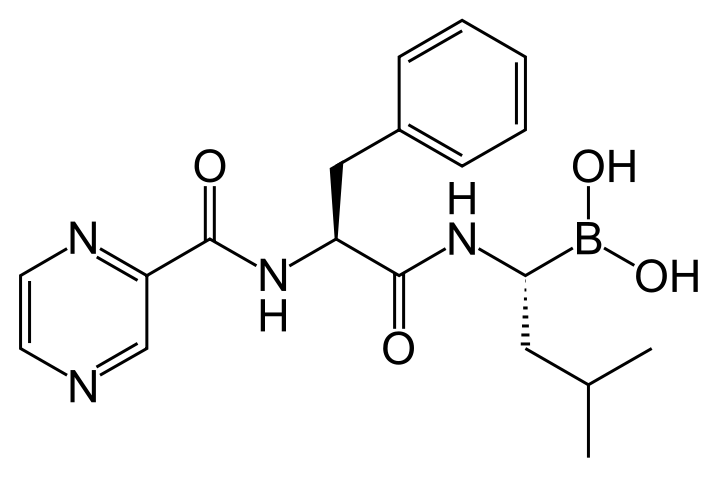
| IUPAC_name = [(1''R'')-3-methyl-1-({(2''S'')-3-phenyl-2-[(pyrazin-2-ylcarbonyl)amino]propanoyl}amino)butyl]boronic acid | |||
| image = Bortezomib.png | |||
| image2 = Bortezomib-from-PDB-2F16-3D-balls.png | |||
<!--Clinical data--> | |||
| tradename = Velcade | |||
| Drugs.com = {{drugs.com|monograph|bortezomib}} | |||
| MedlinePlus = a607007 | |||
| licence_EU = Velcade | |||
| licence_US = Bortezomib | |||
| pregnancy_AU = C | |||
| pregnancy_US = D | |||
| legal_status = Rx-only | |||
| routes_of_administration = [[Intravenous therapy|Intravenous]] | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = | |||
| protein_bound = 83% | |||
| metabolism = [[Liver|Hepatic]], [[Cytochrome P450 oxidase|CYP]] extensively involved | |||
| elimination_half-life = 9 to 15 hours | |||
| excretion = | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 179324-69-7 | |||
| ATC_prefix = L01 | |||
| ATC_suffix = XX32 | |||
| PubChem = 387447 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00188 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 343402 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = 69G8BD63PP | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 325041 | |||
<!--Chemical data--> | |||
| C=19 | H=25 | B=1 | N=4 | O=4 | |||
| molecular_weight = 384.237 g/mol | |||
| smiles = O=C(N[C@H](C(=O)N[C@H](B(O)O)CC(C)C)Cc1ccccc1)c2nccnc2 | |||
| InChI = 1/C19H25BN4O4/c1-13(2)10-17(20(27)28)24-18(25)15(11-14-6-4-3-5-7-14)23-19(26)16-12-21-8-9-22-16/h3-9,12-13,15,17,27-28H,10-11H2,1-2H3,(H,23,26)(H,24,25)/t15-,17-/m0/s1 | |||
| InChIKey = GXJABQQUPOEUTA-RDJZCZTQBH | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C19H25BN4O4/c1-13(2)10-17(20(27)28)24-18(25)15(11-14-6-4-3-5-7-14)23-19(26)16-12-21-8-9-22-16/h3-9,12-13,15,17,27-28H,10-11H2,1-2H3,(H,23,26)(H,24,25)/t15-,17-/m0/s1 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = GXJABQQUPOEUTA-RDJZCZTQSA-N | |||
}} | |||
|mechAction=Bortezomib is a reversible inhibitor of the chymotrypsin-like activity of the 26S proteasome in mammalian cells. The 26S proteasome is a large protein complex that degrades ubiquitinated proteins. The ubiquitin-proteasome pathway plays an essential role in regulating the intracellular concentration of specific proteins, thereby maintaining homeostasis within cells. Inhibition of the 26S proteasome prevents this targeted proteolysis, which can affect multiple signaling cascades within the cell. This disruption of normal homeostatic mechanisms can lead to cell death. Experiments have demonstrated that bortezomib is cytotoxic to a variety of cancer cell types in vitro. Bortezomib causes a delay in tumor growth in vivo in nonclinical tumor models, including multiple myeloma. | |||
|structure=VELCADE® (bortezomib) for Injection is an antineoplastic agent available for intravenous injection or subcutaneous use. Each single use vial contains 3.5 mg of bortezomib as a sterile lyophilized powder. Inactive ingredient: 35 mg mannitol, USP. | |||
Bortezomib is a modified dipeptidyl boronic acid. The product is provided as a mannitol boronic ester which, in reconstituted form, consists of the mannitol ester in equilibrium with its hydrolysis product, the monomeric boronic acid. The drug substance exists in its cyclic anhydride form as a trimeric boroxine. | |||
The chemical name for bortezomib, the monomeric boronic acid, is [(1R)-3-methyl-1-[[(2S)-1-oxo-3-phenyl-2-[(pyrazinylcarbonyl) amino]propyl]amino]butyl] boronic acid. | |||
Bortezomib has the following chemical structure: | |||
[[File:Bortezomib.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
The molecular weight is 384.24. The molecular formula is C19H25BN4O4. The solubility of bortezomib, as the monomeric boronic acid, in water is 3.3 to 3.8 mg/mL in a pH range of 2 to 6.5. | |||
|PD=FDA Package Insert for Bortezomib contains no information regarding Adverse Reactions. | |||
|PK=Following intravenous administration of 1 mg/m2 and 1.3 mg/m2 doses to 24 patients with multiple myeloma (n=12, per each dose level), the mean maximum plasma concentrations of bortezomib (Cmax) after the first dose (Day 1) were 57 and 112 ng/mL, respectively. In subsequent doses, when administered twice weekly, the mean maximum observed plasma concentrations ranged from 67 to 106 ng/mL for the 1 mg/m2 dose and 89 to 120 ng/mL for the 1.3 mg/m2 dose. The mean elimination half-life of bortezomib upon multiple dosing ranged from 40 to 193 hours after the 1 mg/m2 dose and 76 to 108 hours after the 1.3mg/m2 dose. The mean total body clearances was 102 and 112 L/h following the first dose for doses of 1 mg/m2 and 1.3 mg/m2, respectively, and ranged from 15 to 32 L/h following subsequent doses for doses of 1 and 1.3 mg/m2, respectively. | |||
Following an intravenous bolus or subcutaneous injection of a 1.3 mg/m2 dose to patients (n = 14 for intravenous, n = 17 for subcutaneous) with multiple myeloma, the total systemic exposure after repeat dose administration (AUClast) was equivalent for subcutaneous and intravenous administration. The Cmax after subcutaneous administration (20.4 ng/mL) was lower than intravenous (223 ng/mL). The AUClast geometric mean ratio was 0.99 and 90% confidence intervals were 80.18% - 122.80%. | |||
'''Distribution''': The mean distribution volume of bortezomib ranged from approximately 498 to 1884 L/m2 following single- or repeat-dose administration of 1 mg/m2 or 1.3mg/m2 to patients with multiple myeloma. This suggests bortezomib distributes widely to peripheral tissues. The binding of bortezomib to human plasma proteins averaged 83% over the concentration range of 100 to 1000 ng/mL. | |||
Metabolism: In vitro studies with human liver microsomes and human cDNA-expressed cytochrome P450 isozymes indicate that bortezomib is primarily oxidatively metabolized via cytochrome P450 enzymes 3A4, 2C19, and 1A2. Bortezomib metabolism by CYP 2D6 and 2C9 enzymes is minor. The major metabolic pathway is deboronation to form 2 deboronated metabolites that subsequently undergo hydroxylation to several metabolites. Deboronated bortezomib metabolites are inactive as 26S proteasome inhibitors. Pooled plasma data from 8 patients at 10 min and 30 min after dosing indicate that the plasma levels of metabolites are low compared to the parent drug.<br> | |||
'''Elimination''': The pathways of elimination of bortezomib have not been characterized in humans.<br> | |||
'''Age''': Analyses of data after the first dose of Cycle 1 (Day 1) in 39 multiple myeloma patients who had received intravenous doses of 1 mg/m2 and 1.3 mg/m2 showed that both dose-normalized AUC and Cmax tend to be less in younger patients. Patients < 65 years of age (n=26) had about 25% lower mean dose-normalized AUC and Cmax than those ≥ 65 years of age (n=13).<br> | |||
Gender: Mean dose-normalized AUC and Cmax values were comparable between male (n=22) and female (n=17) patients after the first dose of Cycle 1 for the 1 and 1.3 mg/m2 doses.<br> | |||
'''Race''': The effect of race on exposure to bortezomib could not be assessed as most of the patients were Caucasian.<br> | |||
'''Hepatic Impairment''': The effect of hepatic impairment (see Table 4 for definition of hepatic impairment) on the pharmacokinetics of bortezomib was assessed in 60 patients with cancer at bortezomib doses ranging from 0.5 to 1.3 mg/m2. When compared to patients with normal hepatic function, mild hepatic impairment did not alter dose-normalized bortezomib AUC. However, the dose-normalized mean AUC values were increased by approximately 60% in patients with moderate or severe hepatic impairment. A lower starting dose is recommended in patients with moderate or severe hepatic impairment, and those patients should be monitored closely .<br> | |||
'''Renal Impairment''': A pharmacokinetic study was conducted in patients with various degrees of renal impairment who were classified according to their creatinine clearance values (CrCl) into the following groups: Normal (CrCl ≥60 mL/min/1.73 m2, N=12), Mild (CrCl=40-59 mL/min/1.73 m2, N=10), Moderate (CrCl=20-39 mL/min/1.73 m2, N=9), and Severe (CrCl < 20 mL/min/1.73 m2, N=3). A group of dialysis patients who were dosed after dialysis was also included in the study (N=8). Patients were administered intravenous doses of 0.7 to 1.3 mg/m2 of bortezomib twice weekly. Exposure of bortezomib (dose-normalized AUC and Cmax) was comparable among all the groups .<br> | |||
'''Pediatric''': There are no pharmacokinetic data in pediatric patients.<br> | |||
'''Cytochrome P450''': Bortezomib is a poor inhibitor of human liver microsome cytochrome P450 1A2, 2C9, 2D6, and 3A4, with IC50 values of > 30µM (> 11.5µg/mL). Bortezomib may inhibit 2C19 activity (IC50 = 18 µM, 6.9 µg/mL) and increase exposure to drugs that are substrates for this enzyme. Bortezomib did not induce the activities of cytochrome P450 3A4 and 1A2 in primary cultured human hepatocytes. | |||
|nonClinToxic=====Carcinogenesis, Mutagenesis, Impairment of Fertility==== | |||
Carcinogenicity studies have not been conducted with bortezomib. | |||
Bortezomib showed clastogenic activity (structural chromosomal aberrations) in the in vitro chromosomal aberration assay using Chinese hamster ovary cells. Bortezomib was not genotoxic when tested in the in vitro mutagenicity assay (Ames test) and in vivo micronucleus assay in mice. | |||
Fertility studies with bortezomib were not performed but evaluation of reproductive tissues has been performed in the general toxicity studies. In the 6-month rat toxicity study, degenerative effects in the ovary were observed at doses ≥ 0.3 mg/m2 (one-fourth of the recommended clinical dose), and degenerative changes in the testes occurred at 1.2 mg/m2. VELCADE could have a potential effect on either male or female fertility. | |||
====Animal Toxicology and/or Pharmacology==== | |||
<I>Cardiovascular Toxicity</I>: Studies in monkeys showed that administration of dosages approximately twice the recommended clinical dose resulted in heart rate elevations, followed by profound progressive hypotension, bradycardia, and death 12 to 14 hours post dose. Doses ≥ 1.2 mg/m2 induced dose-proportional changes in cardiac parameters. Bortezomib has been shown to distribute to most tissues in the body, including the myocardium. In a repeated dosing toxicity study in the monkey, myocardial hemorrhage, inflammation, and necrosis were also observed. | |||
<I>Chronic Administration</I>: In animal studies at a dose and schedule similar to that recommended for patients (twice weekly dosing for 2 weeks followed by 1-week rest), toxicities observed included severe anemia and thrombocytopenia, and gastrointestinal, neurological and lymphoid system toxicities. Neurotoxic effects of bortezomib in animal studies included axonal swelling and degeneration in peripheral nerves, dorsal spinal roots, and tracts of the spinal cord. Additionally, multifocal hemorrhage and necrosis in the brain, eye, and heart were observed. | |||
|clinicalStudies=====Multiple Myeloma==== | |||
<I>Randomized, Open-Label Clinical Study in Patients with Previously Untreated Multiple Myeloma:</I> | |||
A prospective, international, randomized (1:1), open-label clinical study of 682 patients was conducted to determine whether VELCADE administered intravenously (1.3 mg/m2) in combination with melphalan (9 mg/m2) and prednisone (60 mg/m2) resulted in improvement in time to progression (TTP) when compared to melphalan (9 mg/m2) and prednisone (60 mg/m2) in patients with previously untreated multiple myeloma. Treatment was administered for a maximum of 9 cycles (approximately 54 weeks) and was discontinued early for disease progression or unacceptable toxicity. Antiviral prophylaxis was recommended for patients on the VELCADE study arm. | |||
The median age of the patients in the study was 71 years (48;91), 50% were male, 88% were Caucasian and the median Karnofsky performance status score for the patients was 80 (60;100). Patients had IgG/IgA/Light chain myeloma in 63%/25%/8% instances, a median hemoglobin of 105 g/L (64;165), and a median platelet count of 221,500 /microliter (33,000;587,000). | |||
Efficacy results for the trial are presented in Table 11. At a pre-specified interim analysis (with median follow-up of 16.3 months), the combination of VELCADE, melphalan and prednisone therapy resulted in significantly superior results for time to progression, progression-free survival, overall survival and response rate. Further enrollment was halted, and patients receiving melphalan and prednisone were offered VELCADE in addition. A later, pre-specified analysis of overall survival (with median follow-up of 36.7 months with a hazard ratio of 0.65, 95% CI: 0.51, 0.84) resulted in a statistically significant survival benefit for the VELCADE, melphalan and prednisone treatment arm despite subsequent therapies including VELCADE based regimens. In an updated analysis of overall survival based on 387 deaths (median follow-up 60.1 months), the median overall survival for the VELCADE, melphalan and prednisone treatment arm was 56.4 months and for the melphalan and prednisone treatment arm was 43.1 months, with a hazard ratio of 0.695 (95% CI: 0.57, 0.85). | |||
[[File:bortezomib_clinical studies_01.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
|alcohol=Alcohol-Bortezomib interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Bortezomib interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
}} | }} | ||
Revision as of 18:14, 6 August 2014
{{DrugProjectFormSinglePage |authorTag=Sheng Shi, M.D. [1] |genericName=Bortezomib |aOrAn=a |drugClass=Proteasome Inhibitor |indicationType=treatment |indication=Multiple Myeloma, Mantle Cell Lymphoma |adverseReactions=Hypotension, Rash, Constipation, Decrease in appetite, Diarrhea, Nausea, Vomiting, Anemia, Arthralgia, Bone pain, Cramp, Myalgia, Asthenia, Dizziness, Dysesthesia, Headache, Insomnia, Paresthesia , Peripheral neuropathy, Mental disorder, Cough, Dyspnea, Lower respiratory tract infection, Fever |blackBoxWarningTitle=TITLE |blackBoxWarningBody=Condition Name: (Content)
|fdaLIADAdult=
General Dosing Guidelines
- Recommended starting dosage: 1.3 mg/m2.
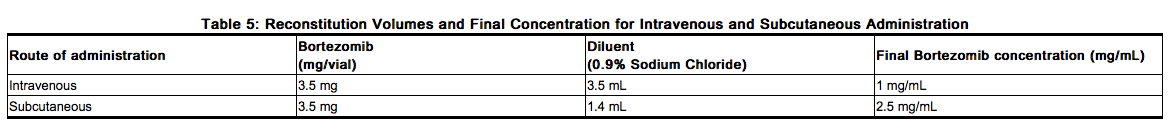
- Administered intravenously at a concentration of 1 mg/mL
- Administered subcutaneously at a concentration of 2.5 mg/mL.
- When administered intravenously, VELCADE is administered as a 3 to 5 second bolus intravenous injection. VELCADE is for intravenous or subcutaneous use only. VELCADE should not be administered by any other route.
- Because each route of administration has a different reconstituted concentration, caution should be used when calculating the volume to be administered.
Dosage in Previously Untreated Multiple Myeloma
- VELCADE is administered in combination with oral melphalan and oral prednisone for nine 6-week treatment cycles as shown in Table 1. In Cycles 1-4, VELCADE is administered twice weekly (days 1, 4, 8, 11, 22, 25, 29 and 32). In Cycles 5-9, VELCADE is administered once weekly (days 1, 8, 22 and 29). At least 72 hours should elapse between consecutive doses of VELCADE.
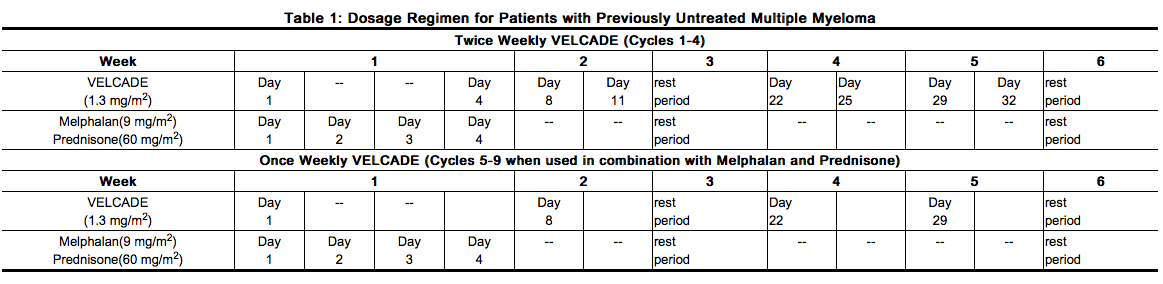
Dose Modification Guidelines for VELCADE When Given in Combination with Melphalan and Prednisone
- Prior to initiating any cycle of therapy with VELCADE in combination with melphalan and prednisone:
- Platelet count should be at least 70 × 109/L and the absolute neutrophil count (ANC) should be at least 1.0 × 109/L
- Non-hematological toxicities should have resolved to Grade 1 or baseline

Dosage and Dose Modifications for Relapsed Multiple Myeloma and Mantle Cell Lymphoma
- VELCADE (1.3 mg/m2/dose) is administered twice weekly for 2 weeks (Days 1, 4, 8, and 11) followed by a 10-day rest period (Days 12-21). For extended therapy of more than 8 cycles, VELCADE may be administered on the standard schedule or on a maintenance schedule of once weekly for 4 weeks (Days 1, 8, 15, and 22) followed by a 13-day rest period (Days 23 to 35) [see Clinical Studies (14)]. At least 72 hours should elapse between consecutive doses of VELCADE.
- VELCADE therapy should be withheld at the onset of any Grade 3 non-hematological or Grade 4 hematological toxicities excluding neuropathy as discussed below [see Warnings and Precautions (5)]. Once the symptoms of the toxicity have resolved, VELCADE therapy may be reinitiated at a 25% reduced dose (1.3 mg/m2/dose reduced to 1 mg/m2/dose; 1 mg/m2/dose reduced to 0.7 mg/m2/dose).
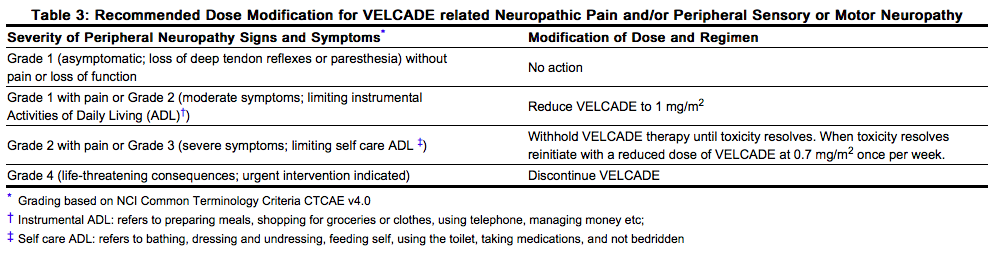
- For dose modifications guidelines for peripheral neuropathy see Management of Peripheral Neuropathy section (2.5).
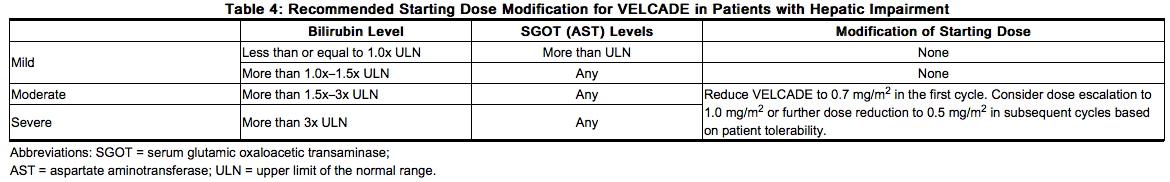
Dosage in Patients with Hepatic Impairment
Patients with mild hepatic impairment do not require a starting dose adjustment and should be treated per the recommended VELCADE dose. Patients with moderate or severe hepatic impairment should be started on VELCADE at a reduced dose of 0.7 mg/m2 per injection during the first cycle, and a subsequent dose escalation to 1.0 mg/m2 or further dose reduction to 0.5 mg/m2 may be considered based on patient tolerance (see Table 4)
Administration Precautions
- The drug quantity contained in one vial (3.5 mg) may exceed the usual dose required. Caution should be used in calculating the dose to prevent overdose.
- When administered subcutaneously, sites for each injection (thigh or abdomen) should be rotated. New injections should be given at least one inch from an old site and never into areas where the site is tender, bruised, erythematous, or indurated.
- If local injection site reactions occur following VELCADE administration subcutaneously, a less concentrated VELCADE solution (1 mg/mL instead of 2.5 mg/mL) may be administered subcutaneously. Alternatively, the intravenous route of administration should be considered
Dose must be individualized to prevent overdosage. After determining patient body surface area (BSA) in square meters, use the following equations to calculate the total volume (mL) of reconstituted VELCADE to be administered:
- Intravenous Administration [1 mg/mL concentration]
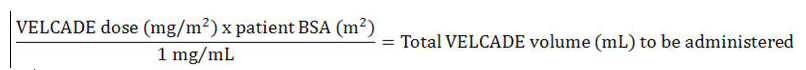
- Subcutaneous Administration [2.5 mg/mL concentration]
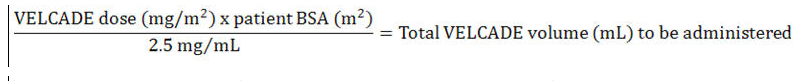
Stickers that indicate the route of administration are provided with each VELCADE vial. These stickers should be placed directly on the syringe of VELCADE once VELCADE is prepared to help alert practitioners of the correct route of administration for VELCADE. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. If any discoloration or particulate matter is observed, the reconstituted product should not be used. Stability: Unopened vials of VELCADE are stable until the date indicated on the package when stored in the original package protected from light. VELCADE contains no antimicrobial preservative. Reconstituted VELCADE should be administered within 8 hours of preparation. When reconstituted as directed, VELCADE may be stored at 25°C (77°F). The reconstituted material may be stored in the original vial and/or the syringe prior to administration. The product may be stored for up to 8 hours in a syringe; however, total storage time for the reconstituted material must not exceed 8 hours when exposed to normal indoor lighting. |offLabelAdultGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Bortezomib in adult patients. |offLabelAdultNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Bortezomib in adult patients. |fdaLIADPed=The safety and effectiveness of VELCADE in children have not been established. |offLabelPedGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Bortezomib in pediatric patients. |offLabelPedNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Bortezomib in pediatric patients. |contraindications=VELCADE is contraindicated in patients with hypersensitivity (not including local reactions) to bortezomib, boron, or mannitol. Reactions have included anaphylactic reactions. VELCADE is contraindicated for intrathecal administration. Fatal events have occurred with intrathecal administration of VELCADE.
|warnings=
Peripheral Neuropathy
VELCADE treatment causes a peripheral neuropathy that is predominantly sensory; however, cases of severe sensory and motor peripheral neuropathy have been reported. Patients with pre-existing symptoms (numbness, pain or a burning feeling in the feet or hands) and/or signs of peripheral neuropathy may experience worsening peripheral neuropathy (including ≥ Grade 3) during treatment with VELCADE. Patients should be monitored for symptoms of neuropathy, such as a burning sensation, hyperesthesia, hypoesthesia, paresthesia, discomfort, neuropathic pain or weakness. In the Phase 3 relapsed multiple myeloma trial comparing VELCADE subcutaneous versus intravenous the incidence of Grade ≥ 2 peripheral neuropathy was 24% for subcutaneous and 39% for intravenous. Grade ≥ 3 peripheral neuropathy occurred in 6% of patients in the subcutaneous treatment group, compared with 15% in the intravenous treatment group. Starting VELCADE subcutaneously may be considered for patients with pre-existing or at high risk of peripheral neuropathy. Patients experiencing new or worsening peripheral neuropathy during VELCADE therapy may require a decrease in the dose and/or a less dose-intense schedule [see Dosage and Administration (2.5)]. In the VELCADE versus dexamethasone phase 3 relapsed multiple myeloma study, improvement in or resolution of peripheral neuropathy was reported in 48% of patients with ≥ Grade 2 peripheral neuropathy following dose adjustment or interruption. Improvement in or resolution of peripheral neuropathy was reported in 73% of patients who discontinued due to Grade 2 neuropathy or who had ≥ Grade 3 peripheral neuropathy in the phase 2 multiple myeloma studies [see Adverse Reactions (6.1)]. The long-term outcome of peripheral neuropathy has not been studied in mantle cell lymphoma.
Hypotension
The incidence of hypotension (postural, orthostatic, and hypotension NOS) was 8%. These events are observed throughout therapy. Caution should be used when treating patients with a history of syncope, patients receiving medications known to be associated with hypotension, and patients who are dehydrated. Management of orthostatic/postural hypotension may include adjustment of antihypertensive medications, hydration, and administration of mineralocorticoids and/or sympathomimetics [see Adverse Reactions (6.1)].
Cardiac Toxicity
Acute development or exacerbation of congestive heart failure and new onset of decreased left ventricular ejection fraction have occurred during VELCADE therapy, including reports in patients with no risk factors for decreased left ventricular ejection fraction. Patients with risk factors for, or existing heart disease should be closely monitored. In the relapsed multiple myeloma study of VELCADE versus dexamethasone, the incidence of any treatment-related cardiac disorder was 8% and 5% in the VELCADE and dexamethasone groups, respectively. The incidence of adverse reactions suggestive of heart failure (acute pulmonary edema, pulmonary edema, cardiac failure, congestive cardiac failure, cardiogenic shock) was ≤ 1% for each individual reaction in the VELCADE group. In the dexamethasone group the incidence was ≤ 1% for cardiac failure and congestive cardiac failure; there were no reported reactions of acute pulmonary edema, pulmonary edema, or cardiogenic shock. There have been isolated cases of QT-interval prolongation in clinical studies; causality has not been established.
Pulmonary Toxicity
Acute Respiratory Distress Syndrome (ARDS) and acute diffuse infiltrative pulmonary disease of unknown etiology such as pneumonitis, interstitial pneumonia, lung infiltration have occurred in patients receiving VELCADE. Some of these events have been fatal. In a clinical trial, the first two patients given high-dose cytarabine (2g/m2 per day) by continuous infusion with daunorubicin and VELCADE for relapsed acute myelogenous leukemia died of ARDS early in the course of therapy. There have been reports of pulmonary hypertension associated with VELCADE administration in the absence of left heart failure or significant pulmonary disease. In the event of new or worsening cardiopulmonary symptoms, consider interrupting VELCADE until a prompt and comprehensive diagnostic evaluation is conducted.
Posterior Reversible Encephalopathy Syndrome (PRES)
Posterior Reversible Encephalopathy Syndrome (PRES; formerly termed Reversible Posterior Leukoencephalopathy Syndrome (RPLS)) has occurred in patients receiving VELCADE. PRES is a rare, reversible, neurological disorder which can present with seizure, hypertension, headache, lethargy, confusion, blindness, and other visual and neurological disturbances. Brain imaging, preferably MRI (Magnetic Resonance Imaging), is used to confirm the diagnosis. In patients developing PRES, discontinue VELCADE. The safety of reinitiating VELCADE therapy in patients previously experiencing PRES is not known.
Gastrointestinal Toxicity
VELCADE treatment can cause nausea, diarrhea, constipation, and vomiting [see Adverse Reactions (6.1)]. sometimes requiring use of antiemetic and antidiarrheal medications. Ileus can occur. Fluid and electrolyte replacement should be administered to prevent dehydration. Interrupt VELCADE for severe symptoms.
Thrombocytopenia/Neutropenia
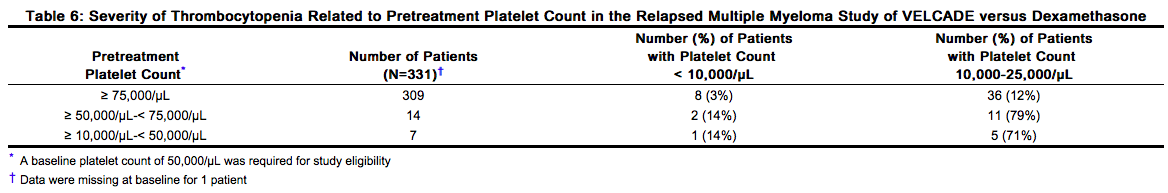
VELCADE is associated with thrombocytopenia and neutropenia that follow a cyclical pattern with nadirs occurring following the last dose of each cycle and typically recovering prior to initiation of the subsequent cycle. The cyclical pattern of platelet and neutrophil decreases and recovery remained consistent over the 8 cycles of twice weekly dosing, and there was no evidence of cumulative thrombocytopenia or neutropenia. The mean platelet count nadir measured was approximately 40% of baseline. The severity of thrombocytopenia related to pretreatment platelet count is shown in Table 6. In the relapsed multiple myeloma study of VELCADE versus dexamethasone, the incidence of bleeding (≥ Grade 3) was 2% on the VELCADE arm and was < 1% in the dexamethasone arm. Complete blood counts (CBC) should be monitored frequently during treatment with VELCADE. Platelet count should be monitored prior to each dose of VELCADE. Patients experiencing thrombocytopenia may require change in the dose and schedule of VELCADE [see Table 2 and Dosage and Administration (2.4)]. Gastrointestinal and intracerebral hemorrhage has been reported in association with VELCADE. Transfusions may be considered.
Tumor Lysis Syndrome
Tumor lysis syndrome has been reported with VELCADE therapy. Patients at risk of tumor lysis syndrome are those with high tumor burden prior to treatment. Monitor patients closely and take appropriate precautions.
Hepatic Toxicity
Cases of acute liver failure have been reported in patients receiving multiple concomitant medications and with serious underlying medical conditions. Other reported hepatic reactions include hepatitis, increases in liver enzymes, and hyperbilirubinemia. Interrupt VELCADE therapy to assess reversibility. There is limited re-challenge information in these patients.
Embryo-fetal Risk
Women of reproductive potential should avoid becoming pregnant while being treated with VELCADE. Bortezomib administered to rabbits during organogenesis at a dose approximately 0.5 times the clinical dose of 1.3 mg/m2 based on body surface area caused post-implantation loss and a decreased number of live fetuses |clinicalTrials=Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Summary of Clinical Trial in Patients with Previously Untreated Multiple Myeloma
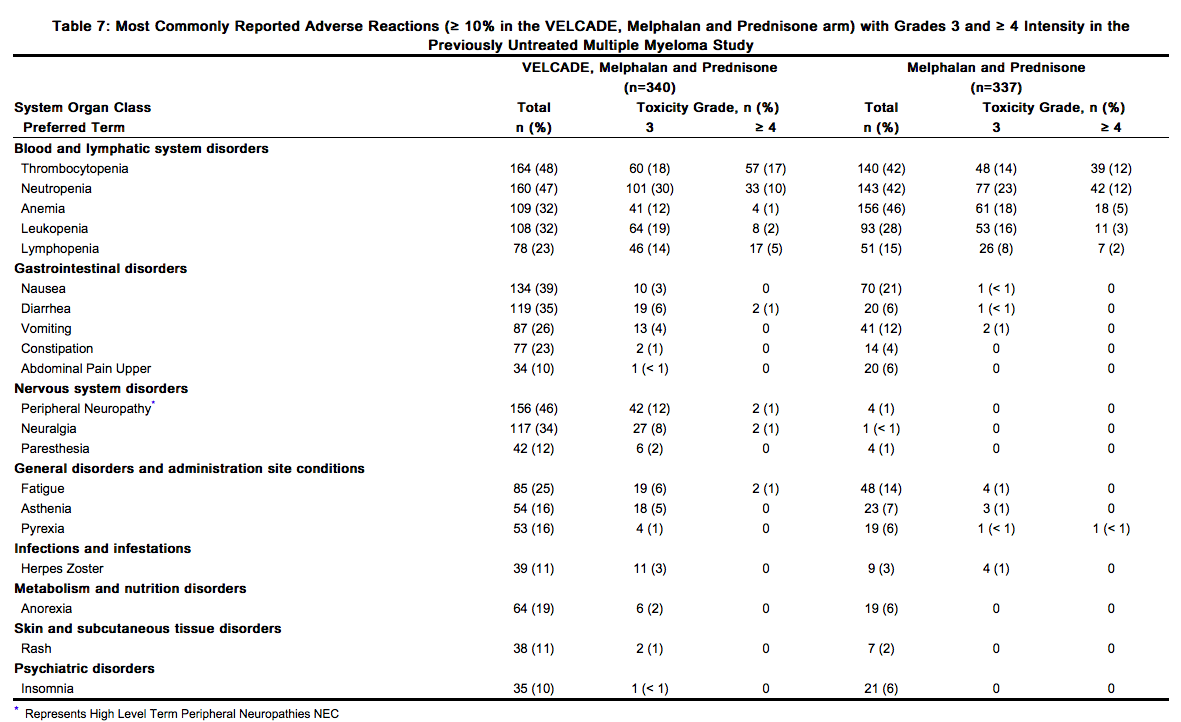
Table 7 describes safety data from 340 patients with previously untreated multiple myeloma who received VELCADE (1.3 mg/m2) administered intravenously in combination with melphalan (9 mg/m2) and prednisone (60 mg/m2) in a prospective randomized study. The safety profile of VELCADE in combination with melphalan/prednisone is consistent with the known safety profiles of both VELCADE and melphalan/prednisone.
‘’‘Relapsed Multiple Myeloma Randomized Study of VELCADE versus Dexamethasone’‘’
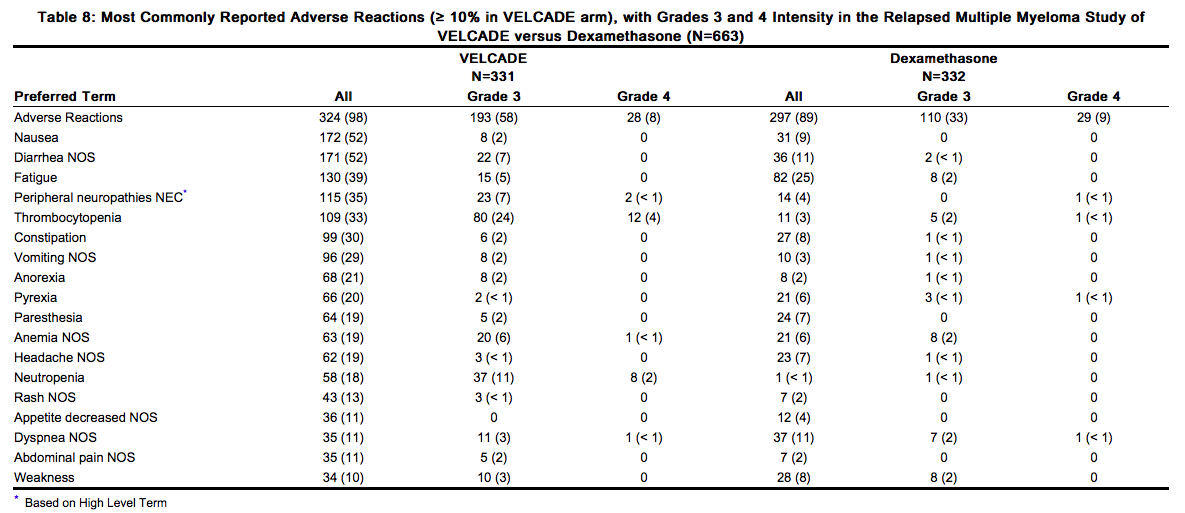
The safety data described below and in Table 8 reflect exposure to either VELCADE (n=331) or dexamethasone (n=332) in a study of patients with relapsed multiple myeloma. VELCADE was administered intravenously at doses of 1.3 mg/m2 twice weekly for 2 out of 3 weeks (21-day cycle). After eight 21-day cycles patients continued therapy for three 35-day cycles on a weekly schedule. Duration of treatment was up to 11 cycles (9 months) with a median duration of 6 cycles (4.1 months). For inclusion in the trial, patients must have had measurable disease and 1 to 3 prior therapies. There was no upper age limit for entry. Creatinine clearance could be as low as 20 mL/min and bilirubin levels as high as 1.5 times the upper limit of normal. The overall frequency of adverse reactions was similar in men and women, and in patients < 65 and ≥ 65 years of age. Most patients were Caucasian [see Clinical Studies (14.1)]. Among the 331 VELCADE-treated patients, the most commonly reported (> 20%) adverse reactions overall were nausea (52%), diarrhea (52%), fatigue (39%), peripheral neuropathies NEC (35%), thrombocytopenia (33%), constipation (30%), vomiting (29%), and anorexia (21%). The most commonly reported (> 20%) adverse reaction reported among the 332 patients in the dexamethasone group was fatigue (25%). Eight percent (8%) of patients in the VELCADE-treated arm experienced a Grade 4 adverse reaction; the most common reactions were thrombocytopenia (4%) and neutropenia (2%). Nine percent (9%) of dexamethasone-treated patients experienced a Grade 4 adverse reaction. All individual dexamethasone-related Grade 4 adverse reactions were less than 1%.
Serious Adverse Reactions and Adverse Reactions Leading to Treatment Discontinuation in the Relapsed Multiple Myeloma Study of VELCADE versus Dexamethasone
Serious adverse reactions are defined as any reaction that results in death, is life-threatening, requires hospitalization or prolongs a current hospitalization, results in a significant disability, or is deemed to be an important medical event. A total of 80 (24%) patients from the VELCADE treatment arm experienced a serious adverse reaction during the study, as did 83 (25%) dexamethasone-treated patients. The most commonly reported serious adverse reactions in the VELCADE treatment arm were diarrhea (3%), dehydration, herpes zoster, pyrexia, nausea, vomiting, dyspnea, and thrombocytopenia (2% each). In the dexamethasone treatment group, the most commonly reported serious adverse reactions were pneumonia (4%), hyperglycemia (3%), pyrexia, and psychotic disorder (2% each). A total of 145 patients, including 84 (25%) of 331 patients in the VELCADE treatment group and 61 (18%) of 332 patients in the dexamethasone treatment group were discontinued from treatment due to adverse reactions. Among the 331 VELCADE treated patients, the most commonly reported adverse reaction leading to discontinuation was peripheral neuropathy (8%). Among the 332 patients in the dexamethasone group, the most commonly reported adverse reactions leading to treatment discontinuation were psychotic disorder and hyperglycemia (2% each). Four deaths were considered to be VELCADE-related in this relapsed multiple myeloma study: 1 case each of cardiogenic shock, respiratory insufficiency, congestive heart failure and cardiac arrest. Four deaths were considered dexamethasone-related: 2 cases of sepsis, 1 case of bacterial meningitis, and 1 case of sudden death at home.
Most Commonly Reported Adverse Reactions in the Relapsed Multiple Myeloma Study of VELCADE versus Dexamethasone
The most common adverse reactions from the relapsed multiple myeloma study are shown in Table 8. All adverse reactions with incidence ≥ 10% in the VELCADE arm are included.
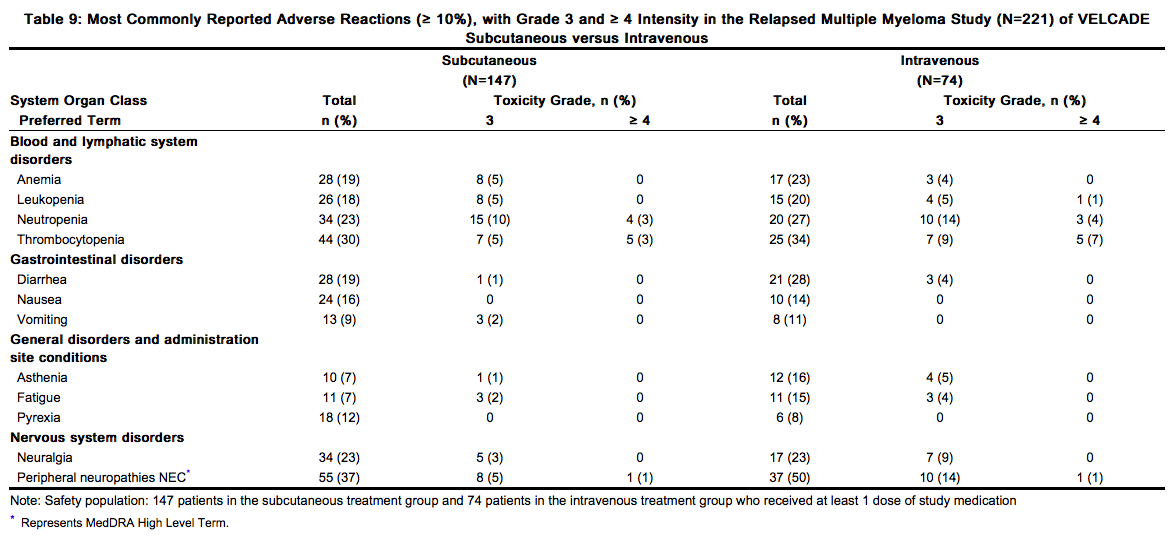
In general, safety data were similar for the subcutaneous and intravenous treatment groups. Differences were observed in the rates of some Grade ≥ 3 adverse reactions. Differences of ≥ 5% were reported in neuralgia (3% subcutaneous versus 9% intravenous), peripheral neuropathies NEC (6% subcutaneous versus 15% intravenous), neutropenia (13% subcutaneous versus 18% intravenous), and thrombocytopenia (8% subcutaneous versus 16% intravenous). A local reaction was reported in 6% of patients in the subcutaneous group, mostly redness. Only 2 (1%) patients were reported as having severe reactions, 1 case of pruritus and 1 case of redness. Local reactions led to reduction in injection concentration in one patient and drug discontinuation in one patient. Local reactions resolved in a median of 6 days. Dose reductions occurred due to adverse reactions in 31% of patients in the subcutaneous treatment group compared with 43% of the intravenously-treated patients. The most common adverse reactions leading to a dose reduction included peripheral sensory neuropathy (17% in the subcutaneous treatment group compared with 31% in the intravenous treatment group); and neuralgia (11% in the subcutaneous treatment group compared with 19% in the intravenous treatment group).
Serious Adverse Reactions and Adverse Reactions Leading to Treatment Discontinuation in the Relapsed Multiple Myeloma Study of VELCADE Subcutaneous versus Intravenous
The incidence of serious adverse reactions was similar for the subcutaneous treatment group (20%) and the intravenous treatment group (19%). The most commonly reported serious adverse reactions in the subcutaneous treatment arm were pneumonia and pyrexia (2% each). In the intravenous treatment group, the most commonly reported serious adverse reactions were pneumonia, diarrhea, and peripheral sensory neuropathy (3% each). In the subcutaneous treatment group, 27 patients (18%) discontinued study treatment due to an adverse reaction compared with 17 patients (23%) in the intravenous treatment group. Among the 147 subcutaneously-treated patients, the most commonly reported adverse reactions leading to discontinuation were peripheral sensory neuropathy (5%) and neuralgia (5%). Among the 74 patients in the intravenous treatment group, the most commonly reported adverse reactions leading to treatment discontinuation were peripheral sensory neuropathy (9%) and neuralgia (9%). Two patients (1%) in the subcutaneous treatment group and 1 (1%) patient in the intravenous treatment group died due to an adverse reaction during treatment. In the subcutaneous group the causes of death were one case of pneumonia and one case of sudden death. In the intravenous group the cause of death was coronary artery insufficiency.
Integrated Summary of Safety (Relapsed Multiple Myeloma and Mantle Cell Lymphoma)
Safety data from phase 2 and 3 studies of single agent VELCADE 1.3 mg/m2/dose twice weekly for 2 weeks followed by a 10-day rest period in 1163 patients with previously-treated multiple myeloma (N=1008) and previously-treated mantle cell lymphoma (N=155) were integrated and tabulated. This analysis does not include data from the Phase 3 Open-Label Study of VELCADE subcutaneous versus intravenous in relapsed multiple myeloma. In the integrated studies, the safety profile of VELCADE was similar in patients with multiple myeloma and mantle cell lymphoma. In the integrated analysis, the most commonly reported (> 20%) adverse reactions were nausea (49%), diarrhea (46%), asthenic conditions including fatigue (41%) and weakness (11%), peripheral neuropathies NEC (38%), thrombocytopenia (32%), vomiting (28%), constipation (25%), and pyrexia (21%). Eleven percent (11%) of patients experienced at least 1 episode of ≥ Grade 4 toxicity, most commonly thrombocytopenia (4%) and neutropenia (2%). In the Phase 2 relapsed multiple myeloma clinical trials of VELCADE administered intravenously, local skin irritation was reported in 5% of patients, but extravasation of VELCADE was not associated with tissue damage.
Serious Adverse Reactions and Adverse Reactions Leading to Treatment Discontinuation in the Integrated Summary of Safety
A total of 26% of patients experienced a serious adverse reaction during the studies. The most commonly reported serious adverse reactions included diarrhea, vomiting and pyrexia (3% each), nausea, dehydration, and thrombocytopenia (2% each) and pneumonia, dyspnea, peripheral neuropathies NEC, and herpes zoster (1% each). Adverse reactions leading to discontinuation occurred in 22% of patients. The reasons for discontinuation included peripheral neuropathy (8%), and fatigue, thrombocytopenia, and diarrhea (2% each). In total, 2% of the patients died and the cause of death was considered by the investigator to be possibly related to study drug: including reports of cardiac arrest, congestive heart failure, respiratory failure, renal failure, pneumonia and sepsis.
Most Commonly Reported Adverse Reactions in the Integrated Summary of Safety
The most common adverse reactions are shown in Table 10. All adverse reactions occurring at ≥ 10% are included. In the absence of a randomized comparator arm, it is often not possible to distinguish between adverse events that are drug-caused and those that reflect the patient's underlying disease. Please see the discussion of specific adverse reactions that follows.
Safety Experience from the Phase 2 Open-Label Extension Study in Relapsed Multiple Myeloma
In the phase 2 extension study of 63 patients, no new cumulative or new long-term toxicities were observed with prolonged VELCADE treatment. These patients were treated for a total of 5.3 to 23 months, including time on VELCADE in the prior VELCADE study [see Clinical Studies (14.1)].
Safety Experience from the Phase 3 Open-Label Study of VELCADE Subcutaneous versus Intravenous in Relapsed Multiple Myeloma
The safety and efficacy of VELCADE administered subcutaneously were evaluated in one Phase 3 study at the recommended dose of 1.3 mg/m2. This was a randomized, comparative study of VELCADE subcutaneous versus intravenous in 222 patients with relapsed multiple myeloma. The safety data described below and in Table 9 reflect exposure to either VELCADE subcutaneous (n=147) or VELCADE intravenous (n=74)
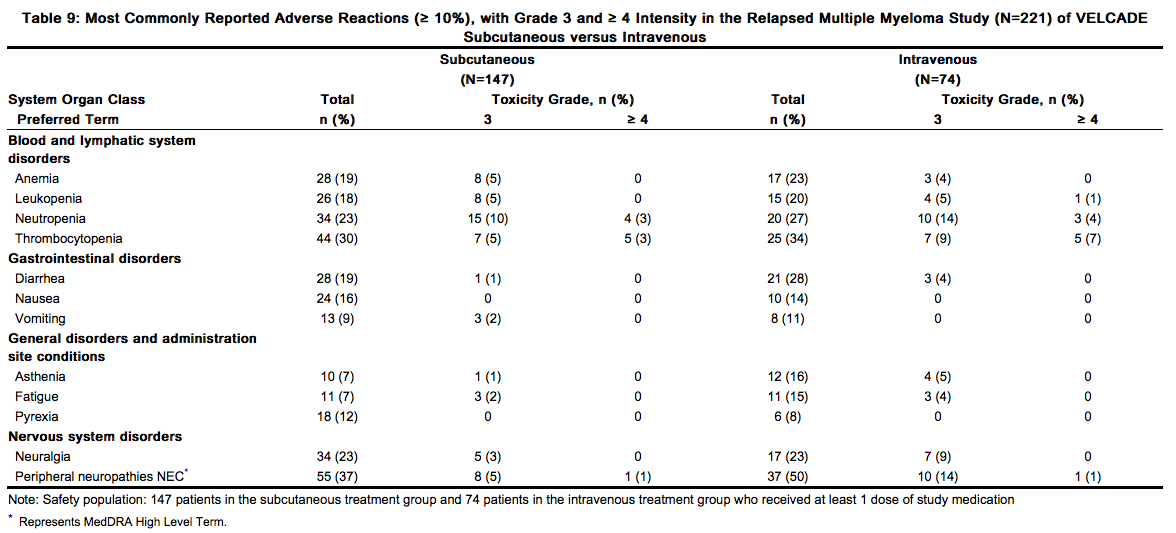
In general, safety data were similar for the subcutaneous and intravenous treatment groups. Differences were observed in the rates of some Grade ≥ 3 adverse reactions. Differences of ≥ 5% were reported in neuralgia (3% subcutaneous versus 9% intravenous), peripheral neuropathies NEC (6% subcutaneous versus 15% intravenous), neutropenia (13% subcutaneous versus 18% intravenous), and thrombocytopenia (8% subcutaneous versus 16% intravenous). A local reaction was reported in 6% of patients in the subcutaneous group, mostly redness. Only 2 (1%) patients were reported as having severe reactions, 1 case of pruritus and 1 case of redness. Local reactions led to reduction in injection concentration in one patient and drug discontinuation in one patient. Local reactions resolved in a median of 6 days. Dose reductions occurred due to adverse reactions in 31% of patients in the subcutaneous treatment group compared with 43% of the intravenously-treated patients. The most common adverse reactions leading to a dose reduction included peripheral sensory neuropathy (17% in the subcutaneous treatment group compared with 31% in the intravenous treatment group); and neuralgia (11% in the subcutaneous treatment group compared with 19% in the intravenous treatment group).
Serious Adverse Reactions and Adverse Reactions Leading to Treatment Discontinuation in the Relapsed Multiple Myeloma Study of VELCADE Subcutaneous versus Intravenous
The incidence of serious adverse reactions was similar for the subcutaneous treatment group (20%) and the intravenous treatment group (19%). The most commonly reported serious adverse reactions in the subcutaneous treatment arm were pneumonia and pyrexia (2% each). In the intravenous treatment group, the most commonly reported serious adverse reactions were pneumonia, diarrhea, and peripheral sensory neuropathy (3% each). In the subcutaneous treatment group, 27 patients (18%) discontinued study treatment due to an adverse reaction compared with 17 patients (23%) in the intravenous treatment group. Among the 147 subcutaneously-treated patients, the most commonly reported adverse reactions leading to discontinuation were peripheral sensory neuropathy (5%) and neuralgia (5%). Among the 74 patients in the intravenous treatment group, the most commonly reported adverse reactions leading to treatment discontinuation were peripheral sensory neuropathy (9%) and neuralgia (9%). Two patients (1%) in the subcutaneous treatment group and 1 (1%) patient in the intravenous treatment group died due to an adverse reaction during treatment. In the subcutaneous group the causes of death were one case of pneumonia and one case of sudden death. In the intravenous group the cause of death was coronary artery insufficiency.
Integrated Summary of Safety (Relapsed Multiple Myeloma and Mantle Cell Lymphoma)
Safety data from phase 2 and 3 studies of single agent VELCADE 1.3 mg/m2/dose twice weekly for 2 weeks followed by a 10-day rest period in 1163 patients with previously-treated multiple myeloma (N=1008) and previously-treated mantle cell lymphoma (N=155) were integrated and tabulated. This analysis does not include data from the Phase 3 Open-Label Study of VELCADE subcutaneous versus intravenous in relapsed multiple myeloma. In the integrated studies, the safety profile of VELCADE was similar in patients with multiple myeloma and mantle cell lymphoma. In the integrated analysis, the most commonly reported (> 20%) adverse reactions were nausea (49%), diarrhea (46%), asthenic conditions including fatigue (41%) and weakness (11%), peripheral neuropathies NEC (38%), thrombocytopenia (32%), vomiting (28%), constipation (25%), and pyrexia (21%). Eleven percent (11%) of patients experienced at least 1 episode of ≥ Grade 4 toxicity, most commonly thrombocytopenia (4%) and neutropenia (2%). In the Phase 2 relapsed multiple myeloma clinical trials of VELCADE administered intravenously, local skin irritation was reported in 5% of patients, but extravasation of VELCADE was not associated with tissue damage.
Serious Adverse Reactions and Adverse Reactions Leading to Treatment Discontinuation in the Integrated Summary of Safety
A total of 26% of patients experienced a serious adverse reaction during the studies. The most commonly reported serious adverse reactions included diarrhea, vomiting and pyrexia (3% each), nausea, dehydration, and thrombocytopenia (2% each) and pneumonia, dyspnea, peripheral neuropathies NEC, and herpes zoster (1% each). Adverse reactions leading to discontinuation occurred in 22% of patients. The reasons for discontinuation included peripheral neuropathy (8%), and fatigue, thrombocytopenia, and diarrhea (2% each). In total, 2% of the patients died and the cause of death was considered by the investigator to be possibly related to study drug: including reports of cardiac arrest, congestive heart failure, respiratory failure, renal failure, pneumonia and sepsis.
Most Commonly Reported Adverse Reactions in the Integrated Summary of Safety
The most common adverse reactions are shown in Table 10. All adverse reactions occurring at ≥ 10% are included. In the absence of a randomized comparator arm, it is often not possible to distinguish between adverse events that are drug-caused and those that reflect the patient's underlying disease. Please see the discussion of specific adverse reactions that follows.
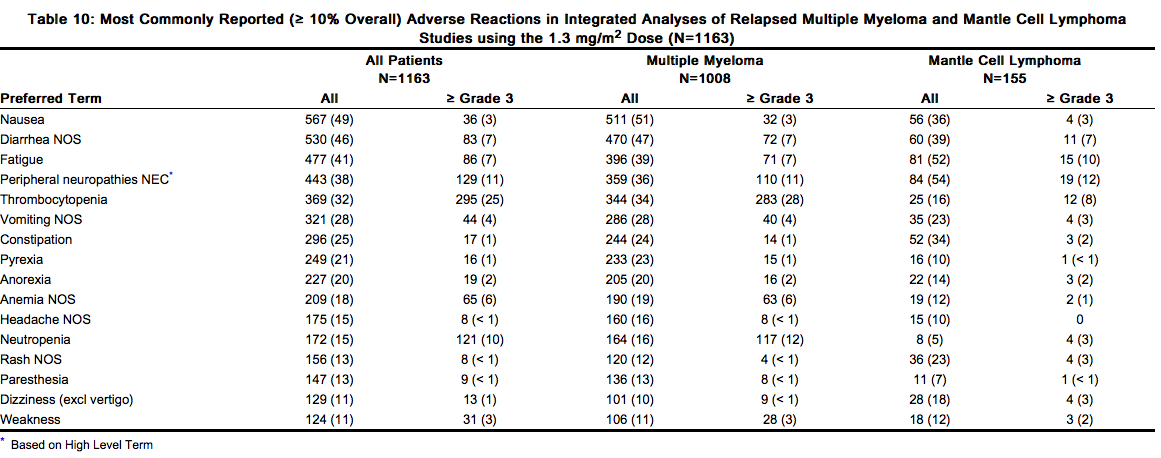
Description of Selected Adverse Reactions from the Integrated Phase 2 and 3 Relapsed Multiple Myeloma and Phase 2 Mantle Cell Lymphoma Studies
Gastrointestinal Toxicity
A total of 75% of patients experienced at least one gastrointestinal disorder. The most common gastrointestinal disorders included nausea, diarrhea, constipation, vomiting, and appetite decreased. Other gastrointestinal disorders included dyspepsia and dysgeusia. Grade 3 adverse reactions occurred in 14% of patients; ≥ Grade 4 adverse reactions were ≤ 1%. Gastrointestinal adverse reactions were considered serious in 7% of patients. Four percent (4%) of patients discontinued due to a gastrointestinal adverse reaction. Nausea was reported more often in patients with multiple myeloma (51%) compared to patients with mantle cell lymphoma (36%).
Thrombocytopenia Across the studies, VELCADE-associated thrombocytopenia was characterized by a decrease in platelet count during the dosing period (days 1 to 11) and a return toward baseline during the 10-day rest period during each treatment cycle. Overall, thrombocytopenia was reported in 32% of patients. Thrombocytopenia was Grade 3 in 22%, ≥ Grade 4 in 4%, and serious in 2% of patients, and the reaction resulted in VELCADE discontinuation in 2% of patients [see Warnings and Precautions (5.7)]. Thrombocytopenia was reported more often in patients with multiple myeloma (34%) compared to patients with mantle cell lymphoma (16%). The incidence of ≥ Grade 3 thrombocytopenia also was higher in patients with multiple myeloma (28%) compared to patients with mantle cell lymphoma (8%).
Peripheral Neuropathy Overall, peripheral neuropathies NEC occurred in 38% of patients. Peripheral neuropathy was Grade 3 for 11% of patients and ≥ Grade 4 for < 1% of patients. Eight percent (8%) of patients discontinued VELCADE due to peripheral neuropathy. The incidence of peripheral neuropathy was higher among patients with mantle cell lymphoma (54%) compared to patients with multiple myeloma (36%). In the VELCADE versus dexamethasone phase 3 relapsed multiple myeloma study, among the 62 VELCADE-treated patients who experienced ≥ Grade 2 peripheral neuropathy and had dose adjustments, 48% had improved or resolved with a median of 3.8 months from first onset. In the phase 2 relapsed multiple myeloma studies, among the 30 patients who experienced Grade 2 peripheral neuropathy resulting in discontinuation or who experienced ≥ Grade 3 peripheral neuropathy, 73% reported improvement or resolution with a median time of 47 days to improvement of one Grade or more from the last dose of VELCADE.
Hypotension
The incidence of hypotension (postural, orthostatic and hypotension NOS) was 8% in patients treated with VELCADE. Hypotension was Grade 1 or 2 in the majority of patients and Grade 3 in 2% and ≥ Grade 4 in < 1%. Two percent (2%) of patients had hypotension reported as a serious adverse reaction, and 1% discontinued due to hypotension. The incidence of hypotension was similar in patients with multiple myeloma (8%) and those with mantle cell lymphoma (9%). In addition, < 1% of patients experienced hypotension associated with a syncopal reaction.
Neutropenia
Neutrophil counts decreased during the VELCADE dosing period (days 1 to 11) and returned toward baseline during the 10-day rest period during each treatment cycle. Overall, neutropenia occurred in 15% of patients and was Grade 3 in 8% of patients and ≥ Grade 4 in 2%. Neutropenia was reported as a serious adverse reaction in < 1% of patients and < 1% of patients discontinued due to neutropenia. The incidence of neutropenia was higher in patients with multiple myeloma (16%) compared to patients with mantle cell lymphoma (5%). The incidence of ≥ Grade 3 neutropenia also was higher in patients with multiple myeloma (12%) compared to patients with mantle cell lymphoma (3%). Asthenic conditions (Fatigue, Malaise, Weakness, Asthenia) Asthenic conditions were reported in 54% of patients. Fatigue was reported as Grade 3 in 7% and ≥ Grade 4 in < 1% of patients. Asthenia was reported as Grade 3 in 2% and ≥ Grade 4 in < 1% of patients. Two percent (2%) of patients discontinued treatment due to fatigue and < 1% due to weakness and asthenia. Asthenic conditions were reported in 53% of patients with multiple myeloma and 59% of patients with mantle cell lymphoma.
Pyrexia
Pyrexia (> 38ºC) was reported as an adverse reaction for 21% of patients. The reaction was Grade 3 in 1% and ≥ Grade 4 in < 1%. Pyrexia was reported as a serious adverse reaction in 3% of patients and led to VELCADE discontinuation in < 1% of patients. The incidence of pyrexia was higher among patients with multiple myeloma (23%) compared to patients with mantle cell lymphoma (10%). The incidence of ≥ Grade 3 pyrexia was 1% in patients with multiple myeloma and < 1% in patients with mantle cell lymphoma.
Herpes Virus Infection
Consider using antiviral prophylaxis in subjects being treated with VELCADE. In the randomized studies in previously untreated and relapsed multiple myeloma, herpes zoster reactivation was more common in subjects treated with VELCADE (ranging between 6-11%) than in the control groups (3-4%). Herpes simplex was seen in 1-3% in subjects treated with VELCADE and 1-3% in the control groups. In the previously untreated multiple myeloma study, herpes zoster virus reactivation in the VELCADE, melphalan and prednisone arm was less common in subjects receiving prophylactic antiviral therapy (3%) than in subjects who did not receive prophylactic antiviral therapy (17%).
Additional Adverse Reactions from Clinical Studies
The following clinically important serious adverse reactions that are not described above have been reported in clinical trials in patients treated with VELCADE administered as monotherapy or in combination with other chemotherapeutics. These studies were conducted in patients with hematological malignancies and in solid tumors.
‘’‘Blood and lymphatic system disorders’‘’: Anemia, disseminated intravascular coagulation, febrile neutropenia, lymphopenia, leukopenia ‘’‘Cardiac disorders’‘’: Angina pectoris, atrial fibrillation aggravated, atrial flutter, bradycardia, sinus arrest, cardiac amyloidosis, complete atrioventricular block, myocardial ischemia, myocardial infarction, pericarditis, pericardial effusion, Torsades de pointes, ventricular tachycardia ‘’‘Ear and labyrinth disorders’‘’: Hearing impaired, vertigo ‘’‘Eye disorders’‘’: Diplopia and blurred vision, conjunctival infection, irritation ‘’‘Gastrointestinal disorders’‘’: Abdominal pain, ascites, dysphagia, fecal impaction, gastroenteritis, gastritis hemorrhagic, hematemesis, hemorrhagic duodenitis, ileus paralytic, large intestinal obstruction, paralytic intestinal obstruction, peritonitis, small intestinal obstruction, large intestinal perforation, stomatitis, melena, pancreatitis acute, oral mucosal petechiae, gastroesophageal reflux ‘’‘General disorders and administration site conditions’‘’: Chills, edema, edema peripheral, injection site erythema, neuralgia, injection site pain, irritation, malaise, phlebitis ‘’‘Hepatobiliary disorders’‘’: Cholestasis, hepatic hemorrhage, hyperbilirubinemia, portal vein thrombosis, hepatitis, liver failure ‘’‘Immune system disorders’‘’: Anaphylactic reaction, drug hypersensitivity, immune complex mediated hypersensitivity, angioedema, laryngeal edema Infections and infestations: Aspergillosis, bacteremia, bronchitis, urinary tract infection, herpes viral infection, listeriosis, nasopharyngitis, pneumonia, respiratory tract infection, septic shock, toxoplasmosis, oral candidiasis, sinusitis, catheter related infection ‘’‘Injury, poisoning and procedural complications’‘’: Catheter related complication, skeletal fracture, subdural hematoma ‘’‘Investigations’‘’: Weight decreased ‘’‘Metabolism and nutrition disorders’‘’: Dehydration, hypocalcemia, hyperuricemia, hypokalemia, hyperkalemia, hyponatremia, hypernatremia ‘’‘Musculoskeletal and connective tissue disorders’‘’: Arthralgia, back pain, bone pain, myalgia, pain in extremity ‘’‘Nervous system disorders’‘’: Ataxia, coma, dizziness, dysarthria, dysesthesia, dysautonomia, encephalopathy, cranial palsy, grand mal convulsion, headache, hemorrhagic stroke, motor dysfunction, neuralgia, spinal cord compression, paralysis, postherpetic neuralgia, transient ischemic attack ‘’‘Psychiatric disorders’‘’: Agitation, anxiety, confusion, insomnia, mental status change, psychotic disorder, suicidal ideation ‘’‘Renal and urinary disorders’‘’: Calculus renal, bilateral hydronephrosis, bladder spasm, hematuria, hemorrhagic cystitis, urinary incontinence, urinary retention, renal failure (acute and chronic), glomerular nephritis proliferative ‘’‘Respiratory, thoracic and mediastinal disorders’‘’: Acute respiratory distress syndrome, aspiration pneumonia, atelectasis, chronic obstructive airways disease exacerbated, cough, dysphagia, dyspnea, dyspnea exertional, epistaxis, hemoptysis, hypoxia, lung infiltration, pleural effusion, pneumonitis, respiratory distress, pulmonary hypertension ‘’‘Skin and subcutaneous tissue disorders’‘’: Urticaria, face edema, rash (which may be pruritic), leukocytoclastic vasculitis, pruritus. ‘’‘Vascular disorders’‘’: Cerebrovascular accident, cerebral hemorrhage, deep venous thrombosis, hypertension, peripheral embolism, pulmonary embolism, pulmonary hypertension |postmarketing=The following adverse reactions have been identified from the worldwide postmarketing experience with VELCADE. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure: atrioventricular block complete, cardiac tamponade, ischemic colitis, encephalopathy, dysautonomia, deafness bilateral, disseminated intravascular coagulation, hepatitis, acute pancreatitis, progressive multifocal leukoencephalopathy (PML), acute diffuse infiltrative pulmonary disease, PRES (formerly RPLS), toxic epidermal necrolysis, acute febrile neutrophilic dermatosis (Sweet's syndrome), herpes meningoencephalitis, optic neuropathy, blindness and ophthalmic herpes. |drugInteractions=Bortezomib is a substrate of cytochrome P450 enzyme 3A4, 2C19 and 1A2.
CYP3A4 inhibitors
Co-administration of ketoconazole, a strong CYP3A4 inhibitor, increased the exposure of bortezomib by 35% in 12 patients. Monitor patients for signs of bortezomib toxicity and consider a bortezomib dose reduction if bortezomib must be given in combination with strong CYP3A4 inhibitors (e.g. ketoconazole, ritonavir).
CYP2C19 inhibitors
Co-administration of omeprazole, a strong inhibitor of CYP2C19, had no effect on the exposure of bortezomib in 17 patients.
CYP3A4 inducers
Co-administration of rifampin, a strong CYP3A4 inducer, is expected to decrease the exposure of bortezomib by at least 45%. Because the drug interaction study (n=6) was not designed to exert the maximum effect of rifampin on bortezomib PK, decreases greater than 45% may occur. Efficacy may be reduced when VELCADE is used in combination with strong CYP3A4 inducers; therefore, concomitant use of strong CYP3A4 inducers is not recommended in patients receiving VELCADE. St. John's Wort (Hypericum perforatum) may decrease bortezomib exposure unpredictably and should be avoided.
Dexamethasone
Co-administration of dexamethasone, a weak CYP3A4 inducer, had no effect on the exposure of bortezomib in 7 patients.
Melphalan-Prednisone
Co-administration of melphalan-prednisone increased the exposure of bortezomib by 17% in 21 patients. However, this increase is unlikely to be clinically relevant. |FDAPregCat=D |useInPregnancyFDA=Bortezomib was not teratogenic in nonclinical developmental toxicity studies in rats and rabbits at the highest dose tested (0.075 mg/kg; 0.5 mg/m2 in the rat and 0.05 mg/kg; 0.6 mg/m2 in the rabbit) when administered during organogenesis. These dosages are approximately half the clinical dose of 1.3 mg/m2 based on body surface area. Pregnant rabbits given bortezomib during organogenesis at a dose of 0.05mg/kg (0.6 mg/m2) experienced significant post-implantation loss and decreased number of live fetuses. Live fetuses from these litters also showed significant decreases in fetal weight. The dose is approximately 0.5 times the clinical dose of 1.3 mg/m2 based on body surface area. There are no adequate and well-controlled studies in pregnant women. If VELCADE is used during pregnancy, or if the patient becomes pregnant while receiving this drug, the patient should be apprised of the potential hazard to the fetus. |useInNursing=It is not known whether bortezomib is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from VELCADE, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. |useInPed=The safety and effectiveness of VELCADE in children have not been established. |useInGeri=Of the 669 patients enrolled in the relapsed multiple myeloma study, 245 (37%) were 65 years of age or older: 125 (38%) on the VELCADE arm and 120 (36%) on the dexamethasone arm. Median time to progression and median duration of response for patients ≥ 65 were longer on VELCADE compared to dexamethasone [5.5 mo versus 4.3 mo, and 8.0 mo versus 4.9 mo, respectively]. On the VELCADE arm, 40% (n=46) of evaluable patients aged ≥ 65 experienced response (CR+PR) versus 18% (n=21) on the dexamethasone arm. The incidence of Grade 3 and 4 events was 64%, 78% and 75% for VELCADE patients ≤ 50, 51-64 and ≥ 65 years old, respectively. No overall differences in safety or effectiveness were observed between patients ≥ age 65 and younger patients receiving VELCADE; but greater sensitivity of some older individuals cannot be ruled out. |useInRenalImpair=The exposure of bortezomib is increased in patients with moderate (bilirubin ≥ 1.5 – 3× ULN) and severe (bilirubin > 3 × ULN) hepatic impairment. Starting dose should be reduced in those patients |useInHepaticImpair=The exposure of bortezomib is increased in patients with moderate (bilirubin ≥ 1.5 – 3× ULN) and severe (bilirubin > 3 × ULN) hepatic impairment. Starting dose should be reduced in those patients |othersTitle=Patients with Diabetes |useInOthers=During clinical trials, hypoglycemia and hyperglycemia were reported in diabetic patients receiving oral hypoglycemics. Patients on oral anti-diabetic agents receiving VELCADE treatment may require close monitoring of their blood glucose levels and adjustment of the dose of their anti-diabetic medication. |administration=intravenous injection or subcutaneous |monitoring=FDA Package Insert for Bortezomib contains no information regarding drug monitoring. |IVCompat=There is limited information about the IV Compatibility. |overdose=There is no known specific antidote for VELCADE overdosage. In humans, fatal outcomes following the administration of more than twice the recommended therapeutic dose have been reported, which were associated with the acute onset of symptomatic hypotension (5.2) and thrombocytopenia (5.7). In the event of an overdosage, the patient's vital signs should be monitored and appropriate supportive care given. Studies in monkeys and dogs showed that intravenous bortezomib doses as low as 2 times the recommended clinical dose on a mg/m2 basis were associated with increases in heart rate, decreases in contractility, hypotension, and death. In dog studies, a slight increase in the corrected QT interval was observed at doses resulting in death. In monkeys, doses of 3.0 mg/m2 and greater (approximately twice the recommended clinical dose) resulted in hypotension starting at 1 hour post-administration, with progression to death in 12 to 14 hours following drug administration. |drugBox=
| |
| |
Bortezomib
| |
| Systematic (IUPAC) name | |
| [(1R)-3-methyl-1-({(2S)-3-phenyl-2-[(pyrazin-2-ylcarbonyl)amino]propanoyl}amino)butyl]boronic acid | |
| Identifiers | |
| CAS number | |
| ATC code | L01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 384.237 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Protein binding | 83% |
| Metabolism | Hepatic, CYP extensively involved |
| Half life | 9 to 15 hours |
| Excretion | ? |
| Therapeutic considerations | |
| Licence data |
, |
| Pregnancy cat. | |
| Legal status |
Template:Unicode Prescription only |
| Routes | Intravenous |
|mechAction=Bortezomib is a reversible inhibitor of the chymotrypsin-like activity of the 26S proteasome in mammalian cells. The 26S proteasome is a large protein complex that degrades ubiquitinated proteins. The ubiquitin-proteasome pathway plays an essential role in regulating the intracellular concentration of specific proteins, thereby maintaining homeostasis within cells. Inhibition of the 26S proteasome prevents this targeted proteolysis, which can affect multiple signaling cascades within the cell. This disruption of normal homeostatic mechanisms can lead to cell death. Experiments have demonstrated that bortezomib is cytotoxic to a variety of cancer cell types in vitro. Bortezomib causes a delay in tumor growth in vivo in nonclinical tumor models, including multiple myeloma. |structure=VELCADE® (bortezomib) for Injection is an antineoplastic agent available for intravenous injection or subcutaneous use. Each single use vial contains 3.5 mg of bortezomib as a sterile lyophilized powder. Inactive ingredient: 35 mg mannitol, USP. Bortezomib is a modified dipeptidyl boronic acid. The product is provided as a mannitol boronic ester which, in reconstituted form, consists of the mannitol ester in equilibrium with its hydrolysis product, the monomeric boronic acid. The drug substance exists in its cyclic anhydride form as a trimeric boroxine. The chemical name for bortezomib, the monomeric boronic acid, is [(1R)-3-methyl-1-[[(2S)-1-oxo-3-phenyl-2-[(pyrazinylcarbonyl) amino]propyl]amino]butyl] boronic acid. Bortezomib has the following chemical structure:

The molecular weight is 384.24. The molecular formula is C19H25BN4O4. The solubility of bortezomib, as the monomeric boronic acid, in water is 3.3 to 3.8 mg/mL in a pH range of 2 to 6.5.
|PD=FDA Package Insert for Bortezomib contains no information regarding Adverse Reactions.
|PK=Following intravenous administration of 1 mg/m2 and 1.3 mg/m2 doses to 24 patients with multiple myeloma (n=12, per each dose level), the mean maximum plasma concentrations of bortezomib (Cmax) after the first dose (Day 1) were 57 and 112 ng/mL, respectively. In subsequent doses, when administered twice weekly, the mean maximum observed plasma concentrations ranged from 67 to 106 ng/mL for the 1 mg/m2 dose and 89 to 120 ng/mL for the 1.3 mg/m2 dose. The mean elimination half-life of bortezomib upon multiple dosing ranged from 40 to 193 hours after the 1 mg/m2 dose and 76 to 108 hours after the 1.3mg/m2 dose. The mean total body clearances was 102 and 112 L/h following the first dose for doses of 1 mg/m2 and 1.3 mg/m2, respectively, and ranged from 15 to 32 L/h following subsequent doses for doses of 1 and 1.3 mg/m2, respectively.
Following an intravenous bolus or subcutaneous injection of a 1.3 mg/m2 dose to patients (n = 14 for intravenous, n = 17 for subcutaneous) with multiple myeloma, the total systemic exposure after repeat dose administration (AUClast) was equivalent for subcutaneous and intravenous administration. The Cmax after subcutaneous administration (20.4 ng/mL) was lower than intravenous (223 ng/mL). The AUClast geometric mean ratio was 0.99 and 90% confidence intervals were 80.18% - 122.80%.
Distribution: The mean distribution volume of bortezomib ranged from approximately 498 to 1884 L/m2 following single- or repeat-dose administration of 1 mg/m2 or 1.3mg/m2 to patients with multiple myeloma. This suggests bortezomib distributes widely to peripheral tissues. The binding of bortezomib to human plasma proteins averaged 83% over the concentration range of 100 to 1000 ng/mL.
Metabolism: In vitro studies with human liver microsomes and human cDNA-expressed cytochrome P450 isozymes indicate that bortezomib is primarily oxidatively metabolized via cytochrome P450 enzymes 3A4, 2C19, and 1A2. Bortezomib metabolism by CYP 2D6 and 2C9 enzymes is minor. The major metabolic pathway is deboronation to form 2 deboronated metabolites that subsequently undergo hydroxylation to several metabolites. Deboronated bortezomib metabolites are inactive as 26S proteasome inhibitors. Pooled plasma data from 8 patients at 10 min and 30 min after dosing indicate that the plasma levels of metabolites are low compared to the parent drug.
Elimination: The pathways of elimination of bortezomib have not been characterized in humans.
Age: Analyses of data after the first dose of Cycle 1 (Day 1) in 39 multiple myeloma patients who had received intravenous doses of 1 mg/m2 and 1.3 mg/m2 showed that both dose-normalized AUC and Cmax tend to be less in younger patients. Patients < 65 years of age (n=26) had about 25% lower mean dose-normalized AUC and Cmax than those ≥ 65 years of age (n=13).
Gender: Mean dose-normalized AUC and Cmax values were comparable between male (n=22) and female (n=17) patients after the first dose of Cycle 1 for the 1 and 1.3 mg/m2 doses.
Race: The effect of race on exposure to bortezomib could not be assessed as most of the patients were Caucasian.
Hepatic Impairment: The effect of hepatic impairment (see Table 4 for definition of hepatic impairment) on the pharmacokinetics of bortezomib was assessed in 60 patients with cancer at bortezomib doses ranging from 0.5 to 1.3 mg/m2. When compared to patients with normal hepatic function, mild hepatic impairment did not alter dose-normalized bortezomib AUC. However, the dose-normalized mean AUC values were increased by approximately 60% in patients with moderate or severe hepatic impairment. A lower starting dose is recommended in patients with moderate or severe hepatic impairment, and those patients should be monitored closely .
Renal Impairment: A pharmacokinetic study was conducted in patients with various degrees of renal impairment who were classified according to their creatinine clearance values (CrCl) into the following groups: Normal (CrCl ≥60 mL/min/1.73 m2, N=12), Mild (CrCl=40-59 mL/min/1.73 m2, N=10), Moderate (CrCl=20-39 mL/min/1.73 m2, N=9), and Severe (CrCl < 20 mL/min/1.73 m2, N=3). A group of dialysis patients who were dosed after dialysis was also included in the study (N=8). Patients were administered intravenous doses of 0.7 to 1.3 mg/m2 of bortezomib twice weekly. Exposure of bortezomib (dose-normalized AUC and Cmax) was comparable among all the groups .
Pediatric: There are no pharmacokinetic data in pediatric patients.
Cytochrome P450: Bortezomib is a poor inhibitor of human liver microsome cytochrome P450 1A2, 2C9, 2D6, and 3A4, with IC50 values of > 30µM (> 11.5µg/mL). Bortezomib may inhibit 2C19 activity (IC50 = 18 µM, 6.9 µg/mL) and increase exposure to drugs that are substrates for this enzyme. Bortezomib did not induce the activities of cytochrome P450 3A4 and 1A2 in primary cultured human hepatocytes.
|nonClinToxic=====Carcinogenesis, Mutagenesis, Impairment of Fertility====
Carcinogenicity studies have not been conducted with bortezomib. Bortezomib showed clastogenic activity (structural chromosomal aberrations) in the in vitro chromosomal aberration assay using Chinese hamster ovary cells. Bortezomib was not genotoxic when tested in the in vitro mutagenicity assay (Ames test) and in vivo micronucleus assay in mice. Fertility studies with bortezomib were not performed but evaluation of reproductive tissues has been performed in the general toxicity studies. In the 6-month rat toxicity study, degenerative effects in the ovary were observed at doses ≥ 0.3 mg/m2 (one-fourth of the recommended clinical dose), and degenerative changes in the testes occurred at 1.2 mg/m2. VELCADE could have a potential effect on either male or female fertility.
Animal Toxicology and/or Pharmacology
Cardiovascular Toxicity: Studies in monkeys showed that administration of dosages approximately twice the recommended clinical dose resulted in heart rate elevations, followed by profound progressive hypotension, bradycardia, and death 12 to 14 hours post dose. Doses ≥ 1.2 mg/m2 induced dose-proportional changes in cardiac parameters. Bortezomib has been shown to distribute to most tissues in the body, including the myocardium. In a repeated dosing toxicity study in the monkey, myocardial hemorrhage, inflammation, and necrosis were also observed. Chronic Administration: In animal studies at a dose and schedule similar to that recommended for patients (twice weekly dosing for 2 weeks followed by 1-week rest), toxicities observed included severe anemia and thrombocytopenia, and gastrointestinal, neurological and lymphoid system toxicities. Neurotoxic effects of bortezomib in animal studies included axonal swelling and degeneration in peripheral nerves, dorsal spinal roots, and tracts of the spinal cord. Additionally, multifocal hemorrhage and necrosis in the brain, eye, and heart were observed. |clinicalStudies=====Multiple Myeloma====
Randomized, Open-Label Clinical Study in Patients with Previously Untreated Multiple Myeloma: A prospective, international, randomized (1:1), open-label clinical study of 682 patients was conducted to determine whether VELCADE administered intravenously (1.3 mg/m2) in combination with melphalan (9 mg/m2) and prednisone (60 mg/m2) resulted in improvement in time to progression (TTP) when compared to melphalan (9 mg/m2) and prednisone (60 mg/m2) in patients with previously untreated multiple myeloma. Treatment was administered for a maximum of 9 cycles (approximately 54 weeks) and was discontinued early for disease progression or unacceptable toxicity. Antiviral prophylaxis was recommended for patients on the VELCADE study arm. The median age of the patients in the study was 71 years (48;91), 50% were male, 88% were Caucasian and the median Karnofsky performance status score for the patients was 80 (60;100). Patients had IgG/IgA/Light chain myeloma in 63%/25%/8% instances, a median hemoglobin of 105 g/L (64;165), and a median platelet count of 221,500 /microliter (33,000;587,000). Efficacy results for the trial are presented in Table 11. At a pre-specified interim analysis (with median follow-up of 16.3 months), the combination of VELCADE, melphalan and prednisone therapy resulted in significantly superior results for time to progression, progression-free survival, overall survival and response rate. Further enrollment was halted, and patients receiving melphalan and prednisone were offered VELCADE in addition. A later, pre-specified analysis of overall survival (with median follow-up of 36.7 months with a hazard ratio of 0.65, 95% CI: 0.51, 0.84) resulted in a statistically significant survival benefit for the VELCADE, melphalan and prednisone treatment arm despite subsequent therapies including VELCADE based regimens. In an updated analysis of overall survival based on 387 deaths (median follow-up 60.1 months), the median overall survival for the VELCADE, melphalan and prednisone treatment arm was 56.4 months and for the melphalan and prednisone treatment arm was 43.1 months, with a hazard ratio of 0.695 (95% CI: 0.57, 0.85).
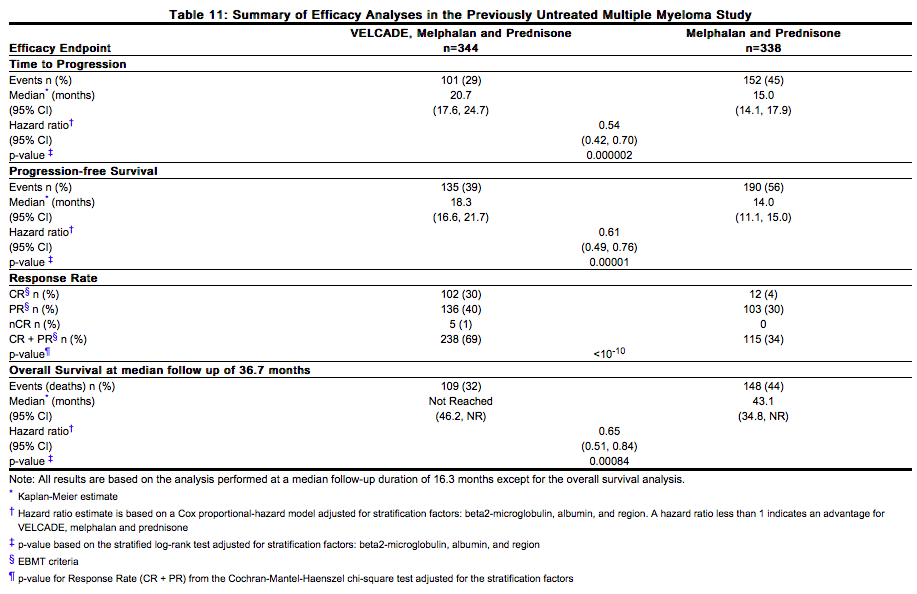
|alcohol=Alcohol-Bortezomib interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
}}