Artesunate: Difference between revisions
m (Protected "Artesunate": Protecting pages from unwanted edits ([edit=sysop] (indefinite) [move=sysop] (indefinite))) |
Rabin Bista (talk | contribs) No edit summary |
||
| (One intermediate revision by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{Drugbox | ||
| | | Verifiedfields = changed | ||
| | | Watchedfields = changed | ||
| | | verifiedrevid = 457818909 | ||
| | | IUPAC_name = | ||
| | | image = Artesunate Wiki Str.png | ||
<!--Clinical data--> | |||
| | | tradename = | ||
| | | Drugs.com = {{drugs.com|international|artesunate}} | ||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | |||
| pregnancy_US = <!-- A / B / C / D / X --> | |||
| | |||
| | |||
| pregnancy_AU = | |||
| pregnancy_US = | |||
| pregnancy_category = | | pregnancy_category = | ||
| legal_AU = | | legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S8 --> | ||
| legal_CA = | | legal_CA = <!-- / Schedule I, II, III, IV, V, VI, VII, VIII --> | ||
| legal_UK = | | legal_UK = | ||
| legal_US = | | legal_US = | ||
| legal_status = Not licensed in UK or US | | legal_status = Not licensed in UK or US | ||
| routes_of_administration = oral, IV, IM | | routes_of_administration = oral, IV, IM | ||
<!--Pharmacokinetic data--> | |||
| bioavailability = | |||
| protein_bound = | |||
| metabolism = | |||
| elimination_half-life = | |||
| excretion = | |||
<!--Identifiers--> | |||
| CAS_number_Ref = {{cascite|changed|??}} | |||
| CAS_number = 88495-63-0 | |||
| CAS_supplemental = <br />{{CAS|80155-81-3}} ([[sodium]] salt) | |||
| ATC_prefix = P01 | |||
| ATC_suffix = BE03 | |||
| PubChem = 5464098 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 16735675 | |||
| NIAID_ChemDB = 112081 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = 60W3249T9M | |||
| ChEMBL_Ref = {{ebicite|changed|EBI}} | |||
| ChEMBL = 258608 | |||
<!--Chemical data--> | |||
| C=19 | H=28 | O=8 | |||
| molecular_weight = 384.421 g/mol | |||
| smiles = [H][C@@]12CC[C@@]3(C)OO[C@@]11[C@@]([H])(CC[C@H]2C)[C@@H](C)[C@H](OC(=O)CCC(O)=O)O[C@]1([H])O3 | |||
| StdInChI_Ref = {{stdinchicite|changed|chemspider}} | |||
| StdInChI = 1S/C19H28O8/c1-10-4-5-13-11(2)16(23-15(22)7-6-14(20)21)24-17-19(13)12(10)8-9-18(3,25-17)26-27-19/h10-13,16-17H,4-9H2,1-3H3,(H,20,21)/t10-,11-,12+,13+,16-,17-,18-,19-/m1/s1 | |||
| StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} | |||
| StdInChIKey = FIHJKUPKCHIPAT-AHIGJZGOSA-N | |||
}} | }} | ||
'''Artesunate''' ([[International Nonproprietary Name|INN]]) is part of the [[artemisinin]] group of drugs that treat malaria. It is a semi-synthetic derivative of artemisinin that is water-soluble and may therefore be given by injection. It is sometimes abbreviated ''' | '''Artesunate''' ([[International Nonproprietary Name|INN]]) is part of the [[artemisinin]] group of drugs that treat malaria. It is a semi-synthetic derivative of artemisinin that is water-soluble and may therefore be given by injection. It is sometimes abbreviated AS. | ||
<!-- Society and culture --> | |||
It is on the [[World Health Organization's List of Essential Medicines]], the most important medications needed in a basic [[health system]].<ref>{{cite web|title=WHO Model List of EssentialMedicines|url=http://apps.who.int/iris/bitstream/10665/93142/1/EML_18_eng.pdf?ua=1|work=World Health Organization|accessdate=22 April 2014|date=October 2013}}</ref> | |||
==Medical uses== | |||
The [[World Health Organization]] recommends intramuscular or intravenous artesunate as the first line treatment for severe malaria.<ref name="WHO 2010">{{cite web|last=World Health Organization|title=Guidelines for the treatment of malaria; Second edition 2010|url=http://whqlibdoc.who.int/publications/2010/9789241547925_eng.pdf|publisher=World Health Organization|accessdate=10 January 2014}}</ref> Artesunate was shown to prevent more deaths from severe malaria than quinine in two large multicentre randomized controlled trials from Africa<ref>{{cite journal | author=Dondorp AL, ''et al.'' | title=Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial | year=2010 | journal=The Lancet | volume=376 | issue=9753 | pages=1647–1657 | doi=10.1016/S0140-6736(10)61924-1 | pmid=21062666 | url=http://www.thelancet.com/journals/lancet/article/PIIS0140-6736%2810%2961924-1/abstract | pmc=3033534}}</ref> and Asia.<ref>{{cite journal | author=South East Asian Quinine Artesunate Malaria Trial (SEAQUAMAT) | title=Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial | year=2005 | journal=The Lancet | volume=366 | issue=9487 | pages=717–725 | doi=10.1016/S0140-6736(05)67176-0 | pmid=16125588 | url=http://www.thelancet.com/journals/lancet/article/PIIS0140-6736%2805%2967176-0/abstract}}</ref> A subsequent systematic review of seven randomized controlled trials found this beneficial effect to be consistent across all trials.<ref>{{cite journal|last=Sinclair|first=D|author2=Donegan, S|author3= Isba, R|author4= Lalloo, DG|title=Artesunate versus quinine for treating severe malaria.|journal=The Cochrane database of systematic reviews|date=Jun 13, 2012|volume=6|pages=CD005967|doi=10.1002/14651858.CD005967.pub4|pmid=22696354}}</ref> | |||
For severe malaria during pregnancy, there is less certainty about the safety of artesunate during the first trimester but artesunate is recommended as first-line therapy during the second and third trimesters.<ref>WHO (2007). [http://malaria.who.int/docs/mip/artemisinin_compounds_pregnancy.pdf Assessment of the safety of artemisinin compounds in pregnancy]. World Health Organization, Geneva.</ref> | |||
Artesunate is also used to treat less severe forms of malaria when it can be given orally, but should always be taken with a second antimalarial such as [[mefloquine]] or [[amodiaquine]] to avoid the development of resistance.<ref name="WHO 2010" /> | |||
While artesunate is used primarily as treatment for [[malaria]], there is some evidence that it may also have some beneficial effects in ''[[Schistosoma haematobium]]'' infection,<ref>{{cite journal | author=Boulangier D, Dieng Y, Cisse B, ''et al.'' | title=Antischistosomal efficacy of artesunate combination therapies administered as curative treatments for malaria attacks | year=2007 | journal=Trans R Soc Trop Med Hyg | volume=101 | issue=2 | pages=113–16 | doi=10.1016/j.trstmh.2006.03.003 | pmid=16765398}}</ref> but this needs confirming in large randomized trials. | |||
==Adverse effects== | |||
Artesunate is generally safe and well-tolerated. The best recognised side effect of the artemesinins that they lower reticulocyte counts.<ref>{{cite journal | author = Clark RL | year = 2012 | title = Effects of artemisinins on reticulocyte count and relationship to possible embryotoxicity in confirmed and unconfirmed malarial patients | journal = Birth defects research. Part A, Clinical and molecular teratology | volume = 94 | issue = 2 | pages = 61–75 | doi = 10.1002/bdra.22868}}</ref> This is not usually of clinical relevance. | |||
== | Delayed haemolysis (occurring around two weeks after treatment) has been observed in patients treated with artesunate for severe malaria.<ref>{{cite journal | author = Rolling T, Agbenyega T, Issifou S, ''et al.'' | title = Delayed hemolysis after treatment with parenteral artesunate in African children with severe malaria—a double-center prospective study. | journal = J Infect Dis | year = 2013 | pmid = 24376273 | url = http://jid.oxfordjournals.org/content/early/2013/12/26/infdis.jit841.long | doi = 10.1093/infdis/jit841 | volume=209 | issue=12 | pages=1921–8}}</ref> Whether or not this haemolysis is due to artesunate, or to the malaria itself is unclear.<ref>{{cite journal | author = Clark RL | title = Hypothesized cause of delayed hemolysis associated with intravenous artesunate. | journal = Med Hypotheses | year = 2013 | doi = 10.1016/j.mehy.2013.11.027 | pmid = 24370269 | volume=82 | issue=2 | pages=167–70}}</ref> | ||
{{Reflist}} | |||
The safety of artesunate in pregnancy is unclear. There is evidence of embryotoxicity in animal models (defects in long bones and ventricular septal defects in the heart in rats and monkeys). However, observational evidence from 123 human first-trimester pregnancies showed no evidence of damage to the fetus.<ref>{{cite journal | author = Clark RL | title = Embryotoxicity of the artemisinin antimalarials and potential consequences for use in women in the first trimester. | journal = Reprod Toxicol | year = 2009 | volume = 28 | issue = 3 | pages = 285–96 | pmid = 19447170 | doi=10.1016/j.reprotox.2009.05.002}}</ref> | |||
==Synthesis== | |||
Artesunate is prepared from [[dihydroartemisinin]] (DHA) by reacting it with [[succinic acid anhydride]] in basic medium. [[Pyridine]] as base/solvent, [[sodium bicarbonate]] in chloroform and catalyst [[4-Dimethylaminopyridine|DMAP]] (''N'',''N''-dimethylaminopyridine) and [[triethylamine]] in 1,2-dichloroethane have been used, with yields of up to 100%. A large scale process involves treatment of DHA in [[dichloromethane]] with a mixture of pyridine, a catalytic amount of DMAP and succinic anhydride. The dichloromethane mixture is stirred for 6–9 h to get artesunate in quantitative yield. The product is further re-crystallized from dichloromethane. alpha-Artesunate is exclusively formed (m.p 135–137˚C). | |||
==Mechanisms of action== | |||
In a [[hematin]] dependent manner, artesunate has been shown to potently inhibit the essential ''[[Plasmodium falciparum]]'' exported protein 1 (EXP1), a membrane [[MAPEG | glutathione S-transferase]].<ref>{{cite pmid| 25126794}}</ref> | |||
==Drug resistance== | |||
Clinical evidence of drug resistance has appeared in Western Cambodia, where artemisinin monotherapy is common.<ref>{{cite journal|author=White NJ|title=Qinghaosu (Artemisinin): The price of success|journal=Science|year=2008|volume=320|issue=5874|pages=330–334|pmid=18420924|doi=10.1126/science.1155165}}</ref> There are as yet no reports of resistance emerging elsewhere. | |||
==References== | |||
{{Reflist|2}} | |||
{{Antimalarials}} | {{Antimalarials}} | ||
[[Category:Antimalarial agents]] | [[Category:Antimalarial agents]] | ||
[[Category: | [[Category:Organic peroxides]] | ||
[[Category:Carboxylate esters]] | |||
[[Category:Sesquiterpenes]] | |||
[[Category:Trioxanes]] | |||
[[ | |||
Latest revision as of 19:43, 6 April 2015
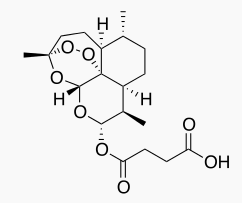 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | oral, IV, IM |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| NIAID ChemDB | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C19H28O8 |
| Molar mass | 384.421 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Artesunate (INN) is part of the artemisinin group of drugs that treat malaria. It is a semi-synthetic derivative of artemisinin that is water-soluble and may therefore be given by injection. It is sometimes abbreviated AS.
It is on the World Health Organization's List of Essential Medicines, the most important medications needed in a basic health system.[1]
Medical uses
The World Health Organization recommends intramuscular or intravenous artesunate as the first line treatment for severe malaria.[2] Artesunate was shown to prevent more deaths from severe malaria than quinine in two large multicentre randomized controlled trials from Africa[3] and Asia.[4] A subsequent systematic review of seven randomized controlled trials found this beneficial effect to be consistent across all trials.[5]
For severe malaria during pregnancy, there is less certainty about the safety of artesunate during the first trimester but artesunate is recommended as first-line therapy during the second and third trimesters.[6]
Artesunate is also used to treat less severe forms of malaria when it can be given orally, but should always be taken with a second antimalarial such as mefloquine or amodiaquine to avoid the development of resistance.[2]
While artesunate is used primarily as treatment for malaria, there is some evidence that it may also have some beneficial effects in Schistosoma haematobium infection,[7] but this needs confirming in large randomized trials.
Adverse effects
Artesunate is generally safe and well-tolerated. The best recognised side effect of the artemesinins that they lower reticulocyte counts.[8] This is not usually of clinical relevance.
Delayed haemolysis (occurring around two weeks after treatment) has been observed in patients treated with artesunate for severe malaria.[9] Whether or not this haemolysis is due to artesunate, or to the malaria itself is unclear.[10]
The safety of artesunate in pregnancy is unclear. There is evidence of embryotoxicity in animal models (defects in long bones and ventricular septal defects in the heart in rats and monkeys). However, observational evidence from 123 human first-trimester pregnancies showed no evidence of damage to the fetus.[11]
Synthesis
Artesunate is prepared from dihydroartemisinin (DHA) by reacting it with succinic acid anhydride in basic medium. Pyridine as base/solvent, sodium bicarbonate in chloroform and catalyst DMAP (N,N-dimethylaminopyridine) and triethylamine in 1,2-dichloroethane have been used, with yields of up to 100%. A large scale process involves treatment of DHA in dichloromethane with a mixture of pyridine, a catalytic amount of DMAP and succinic anhydride. The dichloromethane mixture is stirred for 6–9 h to get artesunate in quantitative yield. The product is further re-crystallized from dichloromethane. alpha-Artesunate is exclusively formed (m.p 135–137˚C).
Mechanisms of action
In a hematin dependent manner, artesunate has been shown to potently inhibit the essential Plasmodium falciparum exported protein 1 (EXP1), a membrane glutathione S-transferase.[12]
Drug resistance
Clinical evidence of drug resistance has appeared in Western Cambodia, where artemisinin monotherapy is common.[13] There are as yet no reports of resistance emerging elsewhere.
References
- ↑ "WHO Model List of EssentialMedicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- ↑ 2.0 2.1 World Health Organization. "Guidelines for the treatment of malaria; Second edition 2010" (PDF). World Health Organization. Retrieved 10 January 2014.
- ↑ Dondorp AL; et al. (2010). "Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial". The Lancet. 376 (9753): 1647–1657. doi:10.1016/S0140-6736(10)61924-1. PMC 3033534. PMID 21062666.
- ↑ South East Asian Quinine Artesunate Malaria Trial (SEAQUAMAT) (2005). "Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial". The Lancet. 366 (9487): 717–725. doi:10.1016/S0140-6736(05)67176-0. PMID 16125588.
- ↑ Sinclair, D; Donegan, S; Isba, R; Lalloo, DG (Jun 13, 2012). "Artesunate versus quinine for treating severe malaria". The Cochrane database of systematic reviews. 6: CD005967. doi:10.1002/14651858.CD005967.pub4. PMID 22696354.
- ↑ WHO (2007). Assessment of the safety of artemisinin compounds in pregnancy. World Health Organization, Geneva.
- ↑ Boulangier D, Dieng Y, Cisse B; et al. (2007). "Antischistosomal efficacy of artesunate combination therapies administered as curative treatments for malaria attacks". Trans R Soc Trop Med Hyg. 101 (2): 113–16. doi:10.1016/j.trstmh.2006.03.003. PMID 16765398.
- ↑ Clark RL (2012). "Effects of artemisinins on reticulocyte count and relationship to possible embryotoxicity in confirmed and unconfirmed malarial patients". Birth defects research. Part A, Clinical and molecular teratology. 94 (2): 61&ndash, 75. doi:10.1002/bdra.22868.
- ↑ Rolling T, Agbenyega T, Issifou S; et al. (2013). "Delayed hemolysis after treatment with parenteral artesunate in African children with severe malaria—a double-center prospective study". J Infect Dis. 209 (12): 1921–8. doi:10.1093/infdis/jit841. PMID 24376273.
- ↑ Clark RL (2013). "Hypothesized cause of delayed hemolysis associated with intravenous artesunate". Med Hypotheses. 82 (2): 167–70. doi:10.1016/j.mehy.2013.11.027. PMID 24370269.
- ↑ Clark RL (2009). "Embryotoxicity of the artemisinin antimalarials and potential consequences for use in women in the first trimester". Reprod Toxicol. 28 (3): 285&ndash, 96. doi:10.1016/j.reprotox.2009.05.002. PMID 19447170.
- ↑ PMID 25126794 (PMID 25126794)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ White NJ (2008). "Qinghaosu (Artemisinin): The price of success". Science. 320 (5874): 330&ndash, 334. doi:10.1126/science.1155165. PMID 18420924.
- Pages with script errors
- CS1 maint: Explicit use of et al.
- CS1 maint: Multiple names: authors list
- Pages with incomplete PMID references
- Template:drugs.com link with non-standard subpage
- Drugs with non-standard legal status
- Articles with changed CASNo identifier
- Articles with changed EBI identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles with changed InChI identifier
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Antimalarial agents
- Organic peroxides
- Carboxylate esters
- Sesquiterpenes
- Trioxanes