Arrhythmogenic right ventricular dysplasia pathophysiology
|
Arrhythmogenic right ventricular dysplasia Microchapters |
|
Differentiating Arrhythmogenic right ventricular dysplasia from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Arrhythmogenic right ventricular dysplasia pathophysiology On the Web |
|
American Roentgen Ray Society Images of Arrhythmogenic right ventricular dysplasia pathophysiology |
|
FDA on Arrhythmogenic right ventricular dysplasia pathophysiology |
|
CDC on Arrhythmogenic right ventricular dysplasia pathophysiology |
|
Arrhythmogenic right ventricular dysplasia pathophysiology in the news |
|
Blogs onArrhythmogenic right ventricular dysplasia pathophysiology |
|
Directions to Hospitals Treating Arrhythmogenic right ventricular dysplasia |
|
Risk calculators and risk factors for Arrhythmogenic right ventricular dysplasia pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
The pathogenesis of ARVD involves apoptosis with fatty and fibrous infiltration of the right ventricular free wall leading to heart failure and ventricular arrhythmias.
Pathophysiology
Apoptosis (programmed cell death) appears to play a large role. It is unclear why only the right ventricle is involved. The disease process starts in the subepicardial region and works its way towards the endocardial surface, leading to transmural involvement (possibly accounting for the aneurysmal dilatation of the RV). Residual myocardium is confined to the subendocardial region and the trabeculae of the RV. These trabeculae may become hypertrophied.
Aneurysmal dilatation is seen in 50% of cases at autopsy. It usually occurs in the diaphragmatic, apical, and infundibular regions (known as the triangle of dysplasia). The left ventricle is involved in 50-67% of individuals. If the left ventricle is involved, it is usually late in the course of disease, and confers a poor prognosis.
There are two pathological patterns seen in ARVD, Fatty infiltration and fibro-fatty infiltration.
Fatty infiltration
The first, fatty infiltration, is confined to the right ventricle. This involves a partial or near-complete substitution of myocardium with fatty tissue without wall thinning. It involves predominantly the apical and infundibular regions of the RV. The left ventricle and ventricular septum are usually spared. No inflammatory infiltrates are seen in fatty infiltration. There is evidence of myocyte (myocardial cell) degeneration and death seen in 50% of cases of fatty infiltration.
Fibro-fatty infiltration
The second, fibro-fatty infiltration, involves replacement of myocytes with fibrofatty tissue. A patchy myocarditis is involved in up to 2/3 of cases, with inflammatory infiltrates (mostly T cells) seen on microscopy. Myocardial atrophy is due to injury and apoptosis. This leads to thinning of the RV free wall (to < 3 mm thickness) Myocytes are replaced with fibrofatty tissue. The regions preferentially involved include the RV inflow tract, the RV outflow tract, and the RV apex. However, the LV free wall may be involved in some cases. Involvement of the ventricular septum is rare. The areas involved are prone to aneurysm formation.
Genetics
A heterozygous mutation in the TGFB3 gene (190230) on chromosome 14q24 is responsible for ARVD1. The pattern of inheritance is autosomal dominant.
ARVD2
(600996), caused by mutation in the RYR2 gene (180902) on chromosome 1q42-q43
ARVD3
(602086), on chromosome 14q12-q22
ARVD4
(602087), on chromosome 2q32.1-q32.3
ARVD5
(604400), caused by mutation in the TMEM43 gene (612048) on chromosome 3p23
ARVD6
(604401), on chromosome 10p14-p12
ARVD7
609160), on chromosome 10q22.3
ARVD8
607450), caused by mutation in the DSP gene (125647) on chromosome 6p24
ARVD9
(609040), caused by mutation in the PKP2 gene (602861) on chromosome 12p11
ARVD10
(610193), caused by mutation in the DSG2 (125671) on chromosome 18q12.1-q12
ARVD11
(610476), caused by mutation in the DSC2 gene (125645) on chromosome 18q12.1
ARVD12
(611528), caused by mutation in the JUP gene (173325) on chromosome 17q21
Ventricular arrhythmias
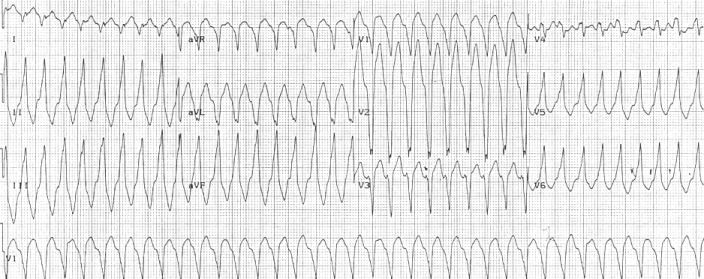 Right ventricular outflow tract tachycardia |
| Monomorphic ventricular tachycardia originating from the right ventricular outflow tract. |
Ventricular arrhythmias due to ARVD typically arise from the diseased right ventricle. The type of arrhythmia ranges from frequent premature ventricular complexes (PVCs) to ventricular tachycardia (VT) to ventricular fibrillation (VF).
While the initiating factor of the ventricular arrhythmias is unclear, it may be due to triggered activity or reentry.
Ventricular arrhythmias are usually exercise-related, suggesting that they are sensitive to catecholamines. The ventricular beats typically have a right axis deviation. Multiple morphologies of ventricular tachycardia may be present in the same individual, suggesting multiple arrhythmogenic foci or pathways.
Right ventricular outflow tract (RVOT) tachycardia is the most common VT seen in individuals with ARVD. In this case, the EKG shows a left bundle branch block (LBBB) morphology with an inferior axis.