Arrhythmogenic right ventricular dysplasia pathophysiology: Difference between revisions
Hudakarman (talk | contribs) |
|||
| (60 intermediate revisions by 3 users not shown) | |||
| Line 4: | Line 4: | ||
==Overview== | ==Overview== | ||
The pathogenesis of ARVD involves [[apoptosis]] with fatty and fibro-fatty infiltration of the right ventricular free wall leading to [[heart failure]] and [[ventricular arrhythmias]]. | The pathogenesis of ARVD involves [[apoptosis]] with fatty and fibro-fatty infiltration of the right ventricular free wall leading to [[heart failure]] and [[ventricular arrhythmias]]. Arrhythmogenic right ventricular cardiomyopathy is considered as a disease of the desmosome. Desmosomes are abundant in the skin and myocardium and their function is to contribute mechanical attachment between cells and work as an important mediator of the intracellular and intercellular signal transduction. Desmosomes are composed of plakoglobin, plakophilins, desmoplakin, desmogleins, and desmocollins. ARVC is usually caused by mutations in genes encoding for desmosomal proteins such as Plakoglobin (JUP), desmoplakin (DSP), plakophilin‐2 (PKP2), desmoglein‐2 (DSG2), and desmocollin‐2 (DSC2). A minority of cases is caused by mutations in nondesmosomal genes. Mode of transmission of Arrhythmogenic right ventricular cardiomyopathy is mostly an autosomal‐dominant trait. Autosomal‐recessive forms are rare, mostly in the cardiocutaneous syndromes such as Carvajal syndrome and Naxos disease. Studied have shown that multiple mutations with digenic heterozygosity are associated with earlier manifestation and more malignant phenotype. | ||
==Pathophysiology== | ==Pathophysiology== | ||
There are two pathological patterns seen in ARVD, fatty infiltration and fibro-fatty infiltration. | There are two pathological patterns seen in ARVD, fatty infiltration and fibro-fatty infiltration. | ||
===Apoptosis=== | ===Apoptosis=== | ||
[[Apoptosis]] (programmed cell death) appears to play a | [[Apoptosis]] (programmed cell death) appears to play a role in the pathogenesis of ARVD and high levels of [[apopain]] have been observed.<ref>Mallat Z, Tedgui A, Fontaliran F et-al. Evidence of apoptosis in arrhythmogenic right ventricular dysplasia. N. Engl. J. Med. 1996;335 (16): 1190-6. {{doi|10.1056/NEJM199610173351604}} - [http://www.ncbi.nlm.nih.gov/pubmed/8815941 Pubmed citation]</ref> It is unclear why only the [[right ventricle]] is involved. The disease process starts in the subepicardial region and works its way towards the endocardial surface, leading to transmural involvement (possibly accounting for the aneurysmal dilatation of the RV). The presence of residual [[myocardium]] is confined to the subendocardial region and the trabeculae of the RV. These trabeculae may become [[Hypertrophy|hypertrophied]]. | ||
===Aneurysmal Dilation of the Right Ventricle=== | ===Aneurysmal Dilation of the Right Ventricle=== | ||
| Line 21: | Line 21: | ||
=== Fibro-fatty infiltration === | === Fibro-fatty infiltration === | ||
The second, fibro-fatty infiltration, involves replacement of myocytes with | The second, fibro-fatty infiltration, involves replacement of [[myocytes]] with fibro-fatty tissue. A patchy [[myocarditis]] is observed in up to 2/3 of cases, with inflammatory infiltrates (mostly [[T cell]]s) seen on microscopy. Myocardial atrophy is due to injury and [[apoptosis]]. This leads to thinning of the RV free wall (to < 3 mm thickness). The regions preferentially involved include the RV inflow tract, the RV outflow tract, and the RV apex. However, the LV free wall may be involved in some cases. Involvement of the ventricular septum is rare. The areas involved are prone to aneurysm formation. | ||
==Genetics== | ==Genetics== | ||
=== | * Arrhythmogenic right ventricular cardiomyopathy is considered as a disease of the desmosome | ||
( | * Desmosomes are abundant in the skin and myocardium and their function is: | ||
**Contribute mechanical attachment between cells | |||
**Works as an important mediator of the intracellular and intercellular signal transduction | |||
* Desmosomes are composed of plakoglobin, plakophilins, desmoplakin, desmogleins, and desmocollins<ref name="pmid193626772">{{cite journal| author=Basso C, Corrado D, Marcus FI, Nava A, Thiene G| title=Arrhythmogenic right ventricular cardiomyopathy. | journal=Lancet | year= 2009 | volume= 373 | issue= 9671 | pages= 1289-300 | pmid=19362677 | doi=10.1016/S0140-6736(09)60256-7 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19362677 }}</ref> | |||
* ARVC is usually caused by mutations in genes encoding for desmosomal proteins: | |||
**Plakoglobin (JUP), desmoplakin (DSP), plakophilin‐2 (PKP2), desmoglein‐2 (DSG2), and desmocollin‐2 (DSC2)<ref name="pmid26320108">{{cite journal| author=Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J | display-authors=etal| title=2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). | journal=Eur Heart J | year= 2015 | volume= 36 | issue= 41 | pages= 2793-2867 | pmid=26320108 | doi=10.1093/eurheartj/ehv316 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=26320108 }}</ref><ref name="pmid21810866">{{cite journal| author=Ackerman MJ, Priori SG, Willems S, Berul C, Brugada R, Calkins H | display-authors=etal| title=HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies: this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). | journal=Europace | year= 2011 | volume= 13 | issue= 8 | pages= 1077-109 | pmid=21810866 | doi=10.1093/europace/eur245 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=21810866 }}</ref> | |||
* A minority of cases is caused by mutations in nondesmosomal genes | |||
*Mode of transmission of Arrhythmogenic right ventricular cardiomyopathy is mostly an autosomal‐dominant trait | |||
*Autosomal‐recessive forms are rare, mostly in the cardiocutaneous syndromes such as:<ref name="pmid21810866" /> | |||
**Carvajal syndrome | |||
**Naxos disease | |||
*Many studies have shown that multiple mutations with compound and digenic heterozygosity were common and were associated with earlier manifestation and more malignant phenotype<ref name="pmid20152563">{{cite journal| author=Xu T, Yang Z, Vatta M, Rampazzo A, Beffagna G, Pilichou K | display-authors=etal| title=Compound and digenic heterozygosity contributes to arrhythmogenic right ventricular cardiomyopathy. | journal=J Am Coll Cardiol | year= 2010 | volume= 55 | issue= 6 | pages= 587-97 | pmid=20152563 | doi=10.1016/j.jacc.2009.11.020 | pmc=2852685 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=20152563 }}</ref><ref name="pmid20400443">{{cite journal| author=Fressart V, Duthoit G, Donal E, Probst V, Deharo JC, Chevalier P | display-authors=etal| title=Desmosomal gene analysis in arrhythmogenic right ventricular dysplasia/cardiomyopathy: spectrum of mutations and clinical impact in practice. | journal=Europace | year= 2010 | volume= 12 | issue= 6 | pages= 861-8 | pmid=20400443 | doi=10.1093/europace/euq104 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=20400443 }}</ref><ref name="pmid24070718">{{cite journal| author=Rigato I, Bauce B, Rampazzo A, Zorzi A, Pilichou K, Mazzotti E | display-authors=etal| title=Compound and digenic heterozygosity predicts lifetime arrhythmic outcome and sudden cardiac death in desmosomal gene-related arrhythmogenic right ventricular cardiomyopathy. | journal=Circ Cardiovasc Genet | year= 2013 | volume= 6 | issue= 6 | pages= 533-42 | pmid=24070718 | doi=10.1161/CIRCGENETICS.113.000288 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=24070718 }}</ref> | |||
*Recently published animal study demonstrated that loss of PKP2 only in adult myocyte was sufficient for the development of an arrhythmia without overt structural disease, that later gives way to an ARVC and finally, a biventricular dilated cardiomyopathy (DCM) | |||
=== | There is an [[autosomal dominant]] pattern of inheritance.<ref>Basso C, Corrado D, Marcus FI et-al. Arrhythmogenic right ventricular cardiomyopathy. Lancet. 2009;373 (9671): 1289-300. {{doi|10.1016/S0140-6736%2809%2960256-7}} - [http://www.ncbi.nlm.nih.gov/pubmed/19362677 Pubmed citation]</ref><ref>Rampazzo A, Nava A, Erne P et-al. A new locus for arrhythmogenic right ventricular cardiomyopathy (ARVD2) maps to chromosome 1q42-q43. Hum. Mol. Genet. 1995;4 (11): 2151-4. Hum. Mol. Genet. (link) - Pubmed citation</ref><ref>Severini GM, Krajinovic M, Pinamonti B et-al. A new locus for arrhythmogenic right ventricular dysplasia on the long arm of chromosome 14. Genomics. 1996;31 (2): 193-200. [http://linkinghub.elsevier.com/retrieve/pii/S0888754396900312 Genomics (link)] - [http://www.ncbi.nlm.nih.gov/pubmed/8824801 Pubmed citation]</ref><ref>Rampazzo A, Nava A, Miorin M et-al. ARVD4, a new locus for arrhythmogenic right ventricular cardiomyopathy, maps to chromosome 2 long arm. Genomics. 1997;45 (2): 259-63. {{doi|10.1006/geno.1997.4927}} - [http://www.ncbi.nlm.nih.gov/pubmed/9344647 Pubmed citation]</ref><ref>Ahmad F, Li D, Karibe A et-al. Localization of a gene responsible for arrhythmogenic right ventricular dysplasia to chromosome 3p23. Circulation. 98 (25): 2791-5. [http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=9860777 Circulation (link)] - [http://www.ncbi.nlm.nih.gov/pubmed/9860777 Pubmed citation]</ref><ref>Li D, Ahmad F, Gardner MJ et-al. The locus of a novel gene responsible for arrhythmogenic right-ventricular dysplasia characterized by early onset and high penetrance maps to chromosome 10p12-p14. Am. J. Hum. Genet. 2000;66 (1): 148-56. {{doi|10.1086/302713}} - [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1288320 Free text at pubmed] - [http://www.ncbi.nlm.nih.gov/pubmed/10631146 Pubmed citation]</ref><ref>Kuhl A, Melberg A, Meinl E et-al. Myofibrillar myopathy with arrhythmogenic right ventricular cardiomyopathy 7: corroboration and narrowing of the critical region on 10q22.3. Eur. J. Hum. Genet. 2008;16 (3): 367-73. {{doi|10.1038/sj.ejhg.5201980}} - [http://www.ncbi.nlm.nih.gov/pubmed/18197198 Pubmed citation]</ref><ref>Rampazzo A, Nava A, Malacrida S et-al. Mutation in human desmoplakin domain binding to plakoglobin causes a dominant form of arrhythmogenic right ventricular cardiomyopathy. Am. J. Hum. Genet. 2002;71 (5): 1200-6. {{doi|10.1086/344208}} - [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC385098 Free text at pubmed] - [http://www.ncbi.nlm.nih.gov/pubmed/12373648 Pubmed citation]</ref><ref>Grossmann KS, Grund C, Huelsken J et-al. Requirement of plakophilin 2 for heart morphogenesis and cardiac junction formation. J. Cell Biol. 2004;167 (1): 149-60. {{doi|10.1083/jcb.200402096}} - [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2172504 Free text at pubmed] - [http://www.ncbi.nlm.nih.gov/pubmed/15479741 Pubmed citation]</ref><ref>Pilichou K, Nava A, Basso C et-al. Mutations in desmoglein-2 gene are associated with arrhythmogenic right ventricular cardiomyopathy. Circulation. 2006;113 (9): 1171-9. {{doi|10.1161/CIRCULATIONAHA.105.583674}} - [http://www.ncbi.nlm.nih.gov/pubmed/16505173 Pubmed citation]</ref><ref>Syrris P, Ward D, Evans A et-al. Arrhythmogenic right ventricular dysplasia/cardiomyopathy associated with mutations in the desmosomal gene desmocollin-2. Am. J. Hum. Genet. 2006;79 (5): 978-84. {{doi|10.1086/509122}} - [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1698574 Free text at pubmed] - [http://www.ncbi.nlm.nih.gov/pubmed/17033975 Pubmed citation]</ref><ref>Asimaki A, Syrris P, Wichter T et-al. A novel dominant mutation in plakoglobin causes arrhythmogenic right ventricular cardiomyopathy. Am. J. Hum. Genet. 2007;81 (5): 964-73. {{doi|10.1086/521633}} - [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2265660 Free text at pubmed] - [http://www.ncbi.nlm.nih.gov/pubmed/17924338 Pubmed citation]</ref><br /> | ||
{| style="border: 0px; font-size: 90%; margin: 3px;" align=center | |||
! style="background: #4479BA; padding: 5px 5px;" rowspan=1 | {{fontcolor|#FFFFFF|Variant}} | |||
=== | ! style="background: #4479BA; padding: 5px 5px;" rowspan=1 | {{fontcolor|#FFFFFF|Associated mutation}} | ||
[http://omim.org/entry/ | |- | ||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold;" rowspan="1;"|[[ARVD1]] | |||
=== | | style="padding: 5px 5px; background: #F5F5F5;" |This variant is due to a heterozygous mutation in the [[TGFB3]] gene on chromosome 14q24 [http://omim.org/entry/190230 190230] | ||
[http://omim.org/entry/ | |- | ||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold;" rowspan="1;"|[[ARVD2]] | |||
== | | style="padding: 5px 5px; background: #F5F5F5;" |Associated with a mutation in the RYR2 gene on chromosome 1q42-q43 [http://omim.org/entry/180902 180902] | ||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold;" rowspan="1;"|[[ARVD3]] | |||
== | | style="padding: 5px 5px; background: #F5F5F5;" |Associated with a mutation in the chromosome 14q12-q22 region [http://omim.org/entry/602086 602086] | ||
[http://omim.org/entry/610193 | |- | ||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold;" rowspan="1;"|[[ARVD4]] | |||
== | | style="padding: 5px 5px; background: #F5F5F5;" |Associated with a mutation in the chromosome 2q32.1-q32.3 region [http://omim.org/entry/602087 602087] | ||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold;" rowspan="1;"|[[ARVD5]] | |||
== | | style="padding: 5px 5px; background: #F5F5F5;" |Associated with a mutation in the TMEM43 gene on chromosome 3p23 region [http://omim.org/entry/604400 604400] | ||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold;" rowspan="1;"|[[ARVD6]] | |||
| style="padding: 5px 5px; background: #F5F5F5;" |Associated with a mutation in the chromosome 10p14-p12 region [http://omim.org/entry/604401 604401] | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold;" rowspan="1;"|[[ARVD7]] | |||
| style="padding: 5px 5px; background: #F5F5F5;" |Associated with a mutation in the chromosome 10q22.3 region [http://omim.org/entry/609160 609160] | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold;" rowspan="1;"|[[ARVD8]] | |||
| style="padding: 5px 5px; background: #F5F5F5;" |Associated with a mutation in the DSP gene on chromosome 6p24 [http://omim.org/entry/607450 607450], [http://omim.org/entry/125647 125647] | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold;" rowspan="1;"|[[ARVD9]] | |||
| style="padding: 5px 5px; background: #F5F5F5;" |Associated with a mutation in the [[PKP2]] gene on chromosome 12p11 [http://omim.org/entry/609040 609040], [http://omim.org/entry/125647 125647] | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold;" rowspan="1;"|[[ARVD10]] | |||
| style="padding: 5px 5px; background: #F5F5F5;" |Associated with a mutation in the [[DSG2]] gene on chromosome 18q12.1-q12 [http://omim.org/entry/610193 610193], [http://omim.org/entry/125671 125671] | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold;" rowspan="1;"|[[ARVD11]] | |||
| style="padding: 5px 5px; background: #F5F5F5;" |Associated with a mutation in the [[DSC2]] gene on chromosome 18q12.1 [http://omim.org/entry/610476 610476], [http://omim.org/entry/125645 125645] | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold;" rowspan="1;"|[[ARVD12]] | |||
| style="padding: 5px 5px; background: #F5F5F5;" |Associated with a mutation in the [[JUP]] gene on chromosome 17q21 [http://omim.org/entry/611528 611528], [http://omim.org/entry/173325 173325] | |||
|- | |||
|} | |||
== Ventricular arrhythmias == | == Ventricular arrhythmias == | ||
| Line 75: | Line 102: | ||
Ventricular arrhythmias are usually exercise-related, suggesting that they are sensitive to catecholamines. The ventricular beats typically have a right axis deviation. Multiple morphologies of ventricular tachycardia may be present in the same individual, suggesting multiple [[arrhythmogenic]] foci or pathways. | Ventricular arrhythmias are usually exercise-related, suggesting that they are sensitive to catecholamines. The ventricular beats typically have a right axis deviation. Multiple morphologies of ventricular tachycardia may be present in the same individual, suggesting multiple [[arrhythmogenic]] foci or pathways. | ||
Right ventricular outflow tract (RVOT) tachycardia is the most common VT seen in individuals with ARVD. In this case, the EKG shows a [[left bundle branch block]] (LBBB) morphology with an inferior axis. | [[Right ventricular outflow tract (RVOT) tachycardia]] is the most common VT seen in individuals with ARVD. In this case, the EKG shows a [[left bundle branch block]] (LBBB) morphology with an inferior axis. | ||
==References== | ==References== | ||
| Line 82: | Line 109: | ||
{{WH}} | {{WH}} | ||
{{WS}} | {{WS}} | ||
[[CME Category::Cardiology]] | |||
[[Category:Cardiology]] | |||
[[Category:Electrophysiology]] | |||
Latest revision as of 21:42, 15 May 2020
|
Arrhythmogenic right ventricular dysplasia Microchapters |
|
Differentiating Arrhythmogenic right ventricular dysplasia from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Arrhythmogenic right ventricular dysplasia pathophysiology On the Web |
|
American Roentgen Ray Society Images of Arrhythmogenic right ventricular dysplasia pathophysiology |
|
FDA on Arrhythmogenic right ventricular dysplasia pathophysiology |
|
CDC on Arrhythmogenic right ventricular dysplasia pathophysiology |
|
Arrhythmogenic right ventricular dysplasia pathophysiology in the news |
|
Blogs onArrhythmogenic right ventricular dysplasia pathophysiology |
|
Directions to Hospitals Treating Arrhythmogenic right ventricular dysplasia |
|
Risk calculators and risk factors for Arrhythmogenic right ventricular dysplasia pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
The pathogenesis of ARVD involves apoptosis with fatty and fibro-fatty infiltration of the right ventricular free wall leading to heart failure and ventricular arrhythmias. Arrhythmogenic right ventricular cardiomyopathy is considered as a disease of the desmosome. Desmosomes are abundant in the skin and myocardium and their function is to contribute mechanical attachment between cells and work as an important mediator of the intracellular and intercellular signal transduction. Desmosomes are composed of plakoglobin, plakophilins, desmoplakin, desmogleins, and desmocollins. ARVC is usually caused by mutations in genes encoding for desmosomal proteins such as Plakoglobin (JUP), desmoplakin (DSP), plakophilin‐2 (PKP2), desmoglein‐2 (DSG2), and desmocollin‐2 (DSC2). A minority of cases is caused by mutations in nondesmosomal genes. Mode of transmission of Arrhythmogenic right ventricular cardiomyopathy is mostly an autosomal‐dominant trait. Autosomal‐recessive forms are rare, mostly in the cardiocutaneous syndromes such as Carvajal syndrome and Naxos disease. Studied have shown that multiple mutations with digenic heterozygosity are associated with earlier manifestation and more malignant phenotype.
Pathophysiology
There are two pathological patterns seen in ARVD, fatty infiltration and fibro-fatty infiltration.
Apoptosis
Apoptosis (programmed cell death) appears to play a role in the pathogenesis of ARVD and high levels of apopain have been observed.[1] It is unclear why only the right ventricle is involved. The disease process starts in the subepicardial region and works its way towards the endocardial surface, leading to transmural involvement (possibly accounting for the aneurysmal dilatation of the RV). The presence of residual myocardium is confined to the subendocardial region and the trabeculae of the RV. These trabeculae may become hypertrophied.
Aneurysmal Dilation of the Right Ventricle
Aneurysmal dilatation of the right ventricle is observed in 50% of cases at autopsy. It usually occurs in the diaphragmatic, apical, and infundibular regions (known as the triangle of dysplasia).
Left Ventricular Involvement
The left ventricle is involved in 50-67% of individuals. If the left ventricle is involved, it is usually late in the course of disease, and confers a poor prognosis.
Fatty infiltration
The first, fatty infiltration, is confined to the right ventricle. This involves a partial or near-complete substitution of myocardium with fatty tissue without wall thinning. It involves predominantly the apical and infundibular regions of the RV. The left ventricle and ventricular septum are usually spared. No inflammatory infiltrates are seen in fatty infiltration. There is evidence of myocyte (myocardial cell) degeneration and death seen in 50% of cases of fatty infiltration.
Fibro-fatty infiltration
The second, fibro-fatty infiltration, involves replacement of myocytes with fibro-fatty tissue. A patchy myocarditis is observed in up to 2/3 of cases, with inflammatory infiltrates (mostly T cells) seen on microscopy. Myocardial atrophy is due to injury and apoptosis. This leads to thinning of the RV free wall (to < 3 mm thickness). The regions preferentially involved include the RV inflow tract, the RV outflow tract, and the RV apex. However, the LV free wall may be involved in some cases. Involvement of the ventricular septum is rare. The areas involved are prone to aneurysm formation.
Genetics
- Arrhythmogenic right ventricular cardiomyopathy is considered as a disease of the desmosome
- Desmosomes are abundant in the skin and myocardium and their function is:
- Contribute mechanical attachment between cells
- Works as an important mediator of the intracellular and intercellular signal transduction
- Desmosomes are composed of plakoglobin, plakophilins, desmoplakin, desmogleins, and desmocollins[2]
- ARVC is usually caused by mutations in genes encoding for desmosomal proteins:
- A minority of cases is caused by mutations in nondesmosomal genes
- Mode of transmission of Arrhythmogenic right ventricular cardiomyopathy is mostly an autosomal‐dominant trait
- Autosomal‐recessive forms are rare, mostly in the cardiocutaneous syndromes such as:[4]
- Carvajal syndrome
- Naxos disease
- Many studies have shown that multiple mutations with compound and digenic heterozygosity were common and were associated with earlier manifestation and more malignant phenotype[5][6][7]
- Recently published animal study demonstrated that loss of PKP2 only in adult myocyte was sufficient for the development of an arrhythmia without overt structural disease, that later gives way to an ARVC and finally, a biventricular dilated cardiomyopathy (DCM)
There is an autosomal dominant pattern of inheritance.[8][9][10][11][12][13][14][15][16][17][18][19]
| Variant | Associated mutation |
|---|---|
| ARVD1 | This variant is due to a heterozygous mutation in the TGFB3 gene on chromosome 14q24 190230 |
| ARVD2 | Associated with a mutation in the RYR2 gene on chromosome 1q42-q43 180902 |
| ARVD3 | Associated with a mutation in the chromosome 14q12-q22 region 602086 |
| ARVD4 | Associated with a mutation in the chromosome 2q32.1-q32.3 region 602087 |
| ARVD5 | Associated with a mutation in the TMEM43 gene on chromosome 3p23 region 604400 |
| ARVD6 | Associated with a mutation in the chromosome 10p14-p12 region 604401 |
| ARVD7 | Associated with a mutation in the chromosome 10q22.3 region 609160 |
| ARVD8 | Associated with a mutation in the DSP gene on chromosome 6p24 607450, 125647 |
| ARVD9 | Associated with a mutation in the PKP2 gene on chromosome 12p11 609040, 125647 |
| ARVD10 | Associated with a mutation in the DSG2 gene on chromosome 18q12.1-q12 610193, 125671 |
| ARVD11 | Associated with a mutation in the DSC2 gene on chromosome 18q12.1 610476, 125645 |
| ARVD12 | Associated with a mutation in the JUP gene on chromosome 17q21 611528, 173325 |
Ventricular arrhythmias
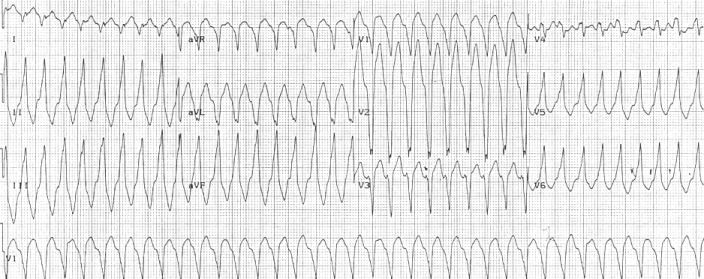 Right ventricular outflow tract tachycardia |
| Monomorphic ventricular tachycardia originating from the right ventricular outflow tract. |
Ventricular arrhythmias due to ARVD typically arise from the diseased right ventricle. The type of arrhythmia ranges from frequent premature ventricular complexes (PVCs) to ventricular tachycardia (VT) to ventricular fibrillation (VF).
While the initiating factor of the ventricular arrhythmias is unclear, it may be due to triggered activity or reentry.
Ventricular arrhythmias are usually exercise-related, suggesting that they are sensitive to catecholamines. The ventricular beats typically have a right axis deviation. Multiple morphologies of ventricular tachycardia may be present in the same individual, suggesting multiple arrhythmogenic foci or pathways.
Right ventricular outflow tract (RVOT) tachycardia is the most common VT seen in individuals with ARVD. In this case, the EKG shows a left bundle branch block (LBBB) morphology with an inferior axis.
References
- ↑ Mallat Z, Tedgui A, Fontaliran F et-al. Evidence of apoptosis in arrhythmogenic right ventricular dysplasia. N. Engl. J. Med. 1996;335 (16): 1190-6. doi:10.1056/NEJM199610173351604 - Pubmed citation
- ↑ Basso C, Corrado D, Marcus FI, Nava A, Thiene G (2009). "Arrhythmogenic right ventricular cardiomyopathy". Lancet. 373 (9671): 1289–300. doi:10.1016/S0140-6736(09)60256-7. PMID 19362677.
- ↑ Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J; et al. (2015). "2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC)". Eur Heart J. 36 (41): 2793–2867. doi:10.1093/eurheartj/ehv316. PMID 26320108.
- ↑ 4.0 4.1 Ackerman MJ, Priori SG, Willems S, Berul C, Brugada R, Calkins H; et al. (2011). "HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies: this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA)". Europace. 13 (8): 1077–109. doi:10.1093/europace/eur245. PMID 21810866.
- ↑ Xu T, Yang Z, Vatta M, Rampazzo A, Beffagna G, Pilichou K; et al. (2010). "Compound and digenic heterozygosity contributes to arrhythmogenic right ventricular cardiomyopathy". J Am Coll Cardiol. 55 (6): 587–97. doi:10.1016/j.jacc.2009.11.020. PMC 2852685. PMID 20152563.
- ↑ Fressart V, Duthoit G, Donal E, Probst V, Deharo JC, Chevalier P; et al. (2010). "Desmosomal gene analysis in arrhythmogenic right ventricular dysplasia/cardiomyopathy: spectrum of mutations and clinical impact in practice". Europace. 12 (6): 861–8. doi:10.1093/europace/euq104. PMID 20400443.
- ↑ Rigato I, Bauce B, Rampazzo A, Zorzi A, Pilichou K, Mazzotti E; et al. (2013). "Compound and digenic heterozygosity predicts lifetime arrhythmic outcome and sudden cardiac death in desmosomal gene-related arrhythmogenic right ventricular cardiomyopathy". Circ Cardiovasc Genet. 6 (6): 533–42. doi:10.1161/CIRCGENETICS.113.000288. PMID 24070718.
- ↑ Basso C, Corrado D, Marcus FI et-al. Arrhythmogenic right ventricular cardiomyopathy. Lancet. 2009;373 (9671): 1289-300. doi:10.1016/S0140-6736%2809%2960256-7 - Pubmed citation
- ↑ Rampazzo A, Nava A, Erne P et-al. A new locus for arrhythmogenic right ventricular cardiomyopathy (ARVD2) maps to chromosome 1q42-q43. Hum. Mol. Genet. 1995;4 (11): 2151-4. Hum. Mol. Genet. (link) - Pubmed citation
- ↑ Severini GM, Krajinovic M, Pinamonti B et-al. A new locus for arrhythmogenic right ventricular dysplasia on the long arm of chromosome 14. Genomics. 1996;31 (2): 193-200. Genomics (link) - Pubmed citation
- ↑ Rampazzo A, Nava A, Miorin M et-al. ARVD4, a new locus for arrhythmogenic right ventricular cardiomyopathy, maps to chromosome 2 long arm. Genomics. 1997;45 (2): 259-63. doi:10.1006/geno.1997.4927 - Pubmed citation
- ↑ Ahmad F, Li D, Karibe A et-al. Localization of a gene responsible for arrhythmogenic right ventricular dysplasia to chromosome 3p23. Circulation. 98 (25): 2791-5. Circulation (link) - Pubmed citation
- ↑ Li D, Ahmad F, Gardner MJ et-al. The locus of a novel gene responsible for arrhythmogenic right-ventricular dysplasia characterized by early onset and high penetrance maps to chromosome 10p12-p14. Am. J. Hum. Genet. 2000;66 (1): 148-56. doi:10.1086/302713 - Free text at pubmed - Pubmed citation
- ↑ Kuhl A, Melberg A, Meinl E et-al. Myofibrillar myopathy with arrhythmogenic right ventricular cardiomyopathy 7: corroboration and narrowing of the critical region on 10q22.3. Eur. J. Hum. Genet. 2008;16 (3): 367-73. doi:10.1038/sj.ejhg.5201980 - Pubmed citation
- ↑ Rampazzo A, Nava A, Malacrida S et-al. Mutation in human desmoplakin domain binding to plakoglobin causes a dominant form of arrhythmogenic right ventricular cardiomyopathy. Am. J. Hum. Genet. 2002;71 (5): 1200-6. doi:10.1086/344208 - Free text at pubmed - Pubmed citation
- ↑ Grossmann KS, Grund C, Huelsken J et-al. Requirement of plakophilin 2 for heart morphogenesis and cardiac junction formation. J. Cell Biol. 2004;167 (1): 149-60. doi:10.1083/jcb.200402096 - Free text at pubmed - Pubmed citation
- ↑ Pilichou K, Nava A, Basso C et-al. Mutations in desmoglein-2 gene are associated with arrhythmogenic right ventricular cardiomyopathy. Circulation. 2006;113 (9): 1171-9. doi:10.1161/CIRCULATIONAHA.105.583674 - Pubmed citation
- ↑ Syrris P, Ward D, Evans A et-al. Arrhythmogenic right ventricular dysplasia/cardiomyopathy associated with mutations in the desmosomal gene desmocollin-2. Am. J. Hum. Genet. 2006;79 (5): 978-84. doi:10.1086/509122 - Free text at pubmed - Pubmed citation
- ↑ Asimaki A, Syrris P, Wichter T et-al. A novel dominant mutation in plakoglobin causes arrhythmogenic right ventricular cardiomyopathy. Am. J. Hum. Genet. 2007;81 (5): 964-73. doi:10.1086/521633 - Free text at pubmed - Pubmed citation