Amikacin sulfate: Difference between revisions
No edit summary |
No edit summary |
||
| Line 17: | Line 17: | ||
*Serious complicated and recurrent [[urinary tract infections]] | *Serious complicated and recurrent [[urinary tract infections]] | ||
|hasBlackBoxWarning=Yes | |hasBlackBoxWarning=Yes | ||
|adverseReactions=[[neuromuscular blockade]], [[ototoxicity]], [[nephrotoxicity]] and [[respiratory paralysis]]. | |adverseReactions=[[neuromuscular blockade]], [[ototoxicity]], [[nephrotoxicity]] and [[respiratory paralysis]]. | ||
|blackBoxWarningTitle=<span style="color:#FF0000;">Warning</span> | |blackBoxWarningTitle=<span style="color:#FF0000;">Warning</span> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> Patients treated with parenteral aminoglycosides should be under close clinical observation because of the potential ototoxicity and nephrotoxicity associated with their use. Safety for treatment periods which are longer than 14 days has not been established. | |blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> Patients treated with parenteral aminoglycosides should be under close clinical observation because of the potential ototoxicity and nephrotoxicity associated with their use. Safety for treatment periods which are longer than 14 days has not been established. | ||
| Line 38: | Line 38: | ||
*[[Joint infections]] | *[[Joint infections]] | ||
*Central nervous system infections (including [[meningitis]]) | *Central nervous system infections (including [[meningitis]]) | ||
**Dosage: | |||
***IV: 15 mg/kg/day IV divided in doses q8h. | |||
***Intraventricular: 5mg-50 mg/day. | |||
*Skin and soft tissue infections | *Skin and soft tissue infections | ||
*Intra-abdominal infections (including [[peritonitis]]). | *Intra-abdominal infections (including [[peritonitis]]). | ||
| Line 59: | Line 62: | ||
*Reduced Dosage at Fixed Time Intervals: When renal function is impaired and it is desirable to administer amikacin at a fixed time interval, dosage must be reduced. In these patients, serum amikacin concentrations should be measured to assure accurate administration of amikacin and to avoid concentrations above 35 mcg/mL. If serum assay determinations are not available and the patient's condition is stable, serum creatinine and creatinine clearance values are the most readily available indicators of the degree of renal impairment to use as a guide for dosage. First, initiate therapy by administering a normal dose, 7.5 mg/kg, as a loading dose. This loading dose is the same as the normally recommended dose which would be calculated for a patient with a normal renal function as described above. To determine the size of maintenance doses administered every 12 hours, the loading dose should be reduced in proportion to the reduction in the patient's creatinine clearance rate: | *Reduced Dosage at Fixed Time Intervals: When renal function is impaired and it is desirable to administer amikacin at a fixed time interval, dosage must be reduced. In these patients, serum amikacin concentrations should be measured to assure accurate administration of amikacin and to avoid concentrations above 35 mcg/mL. If serum assay determinations are not available and the patient's condition is stable, serum creatinine and creatinine clearance values are the most readily available indicators of the degree of renal impairment to use as a guide for dosage. First, initiate therapy by administering a normal dose, 7.5 mg/kg, as a loading dose. This loading dose is the same as the normally recommended dose which would be calculated for a patient with a normal renal function as described above. To determine the size of maintenance doses administered every 12 hours, the loading dose should be reduced in proportion to the reduction in the patient's creatinine clearance rate: | ||
[[file:Formula.png|thumb|center|500px]] | [[file:Formula.png|thumb|center|500px]] | ||
|offLabelAdultGuideSupport= | |||
|offLabelAdultNoGuideSupport= | ======Intravenous Administrations====== | ||
The individual dose, the total daily dose, and the total cumulative dose of amikacin sulfate are identical to the dose recommended for intramuscular administration. The solution for intravenous use is prepared by adding the contents of a 500 mg vial to 100 or 200 mL of sterile diluent such as 0.9% sodium chloride injection or 5% dextrose injection or any other compatible solutions listed below. The solution is administered to adults over a 30 to 60 minute period. The total daily dose should not exceed 15 mg/kg/day and may be divided into either 2 or 3 equally-divided doses at equally-divided intervals. | |||
In pediatric patients, the amount of fluid used will depend on the amount of amikacin sulfate ordered for the patient. It should be a sufficient amount to infuse the amikacin over a 30 to 60 minute period. Infants should receive a 1 to 2 hour infusion. | |||
Amikacin should not be physically premixed with other drugs but should be administered separately according to the recommended dose and route. | |||
|offLabelAdultGuideSupport======Febrile Neutropenia===== | |||
*2010 Guideline - Treatment of febrile neutropenia in cancer patients <ref>{{cite web|url=http://www.uphs.upenn.edu/bugdrug/antibiotic_manual/idsaneutropenicfever2010.pdf|title=Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america}}</ref> | |||
|offLabelAdultNoGuideSupport======Bacterial Endocarditis===== | |||
*Dosage: Amikacin<ref name="pmid7805683">{{cite journal| author=Torres-Tortosa M, de Cueto M, Vergara A, Sánchez-Porto A, Pérez-Guzmán E, González-Serrano M et al.| title=Prospective evaluation of a two-week course of intravenous antibiotics in intravenous drug addicts with infective endocarditis. Grupo de Estudio de Enfermedades Infecciosas de la Provincia de Cádiz. | journal=Eur J Clin Microbiol Infect Dis | year= 1994 | volume= 13 | issue= 7 | pages= 559-64 | pmid=7805683 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=7805683 }} </ref> 7.5 mg/kg/12h + [[Cloxacillin]] 2 g/4h (Duration of therapy: 2 weeks). | |||
=====Eye Infection===== | |||
*Dosage: 25 mg subconjunctival | |||
=====Febrile Neutropenia===== | |||
|fdaLIADPed======Bacterial Meningitis===== | |||
*Dosage: | |||
**IV: 15 mg/kg/day IV divided in doses q8h. | |||
**Intraventricular: 5mg-50 mg/day. | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Amikacin sulfate in pediatric patients. | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Amikacin sulfate in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Amikacin sulfate in pediatric patients. | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Amikacin sulfate in pediatric patients. | ||
|alcohol=Alcohol-Amikacin sulfate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Amikacin sulfate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
}} | }} | ||
Revision as of 20:30, 23 December 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Warning
See full prescribing information for complete Boxed Warning.
Condition Name: Patients treated with parenteral aminoglycosides should be under close clinical observation because of the potential ototoxicity and nephrotoxicity associated with their use. Safety for treatment periods which are longer than 14 days has not been established.
Neurotoxicity, manifested as vestibular and permanent bilateral auditory ototoxicity, can occur in patients with preexisting renal damage and in patients with normal renal function treated at higher doses and/or for periods longer than those recommended. The risk of aminoglycoside-induced ototoxicity is greater in patients with renal damage. High frequency deafness usually occurs first and can be detected only by audiometric testing. Vertigo may occur and may be evidence of vestibular injury. Other manifestations of neurotoxicity may include numbness, skin tingling, muscle twitching and convulsions. The risk of hearing loss due to aminoglycosides increases with the degree of exposure to either high peak or high trough serum concentrations. Patients developing cochlear damage may not have symptoms during therapy to warn them of developing eighth-nerve toxicity, and total or partial irreversible bilateral deafness may occur after the drug has been discontinued. Aminoglycoside-induced ototoxicity is usually irreversible. Aminoglycosides are potentially nephrotoxic. The risk of nephrotoxicity is greater in patients with impaired renal function and in those who receive high doses or prolonged therapy. Neuromuscular blockade and respiratory paralysis have been reported following parenteral injection, topical instillation (as in orthopedic and abdominal irrigation or in local treatment of empyema), and following oral use of aminoglycosides. The possibility of these phenomena should be considered if aminoglycosides are administered by any route, especially in patients receiving anesthetics; neuromuscular blocking agents such as tubocurarine, succinylcholine, decamethonium; or in patients receiving massive transfusions of citrate-anticoagulated blood. If blockage occurs, calcium salts may reverse these phenomena, but mechanical respiratory assistance may be necessary. Renal and eighth-nerve function should be closely monitored especially in patients with known or suspected renal impairment at the onset of therapy and also in those whose renal function is initially normal but who develop signs of renal dysfunction during therapy. Serum concentrations of amikacin should be monitored when feasible to assure adequate levels and to avoid potentially toxic levels and prolonged peak concentrations above 35 micrograms per mL. Urine should be examined for decreased specific gravity, increased excretion of proteins and the presence of cells or casts. Blood urea nitrogen, serum creatinine or creatinine clearance should be measured periodically. Serial audiograms should be obtained where feasible in patients old enough to be tested, particularly high risk patients. Evidence of ototoxicity (dizziness, vertigo, tinnitus, roaring in the ears and hearing loss) or nephrotoxicity requires discontinuation of the drug or dosage adjustment. Concurrent and/or sequential systemic, oral or topical use of other neurotoxic or nephrotoxic products, particularly bacitracin, cisplatin, amphotericin B, cephaloridine, paromomycin, viomycin, polymyxin B, colistin, vancomycin or other aminoglycosides should be avoided. Other factors that may increase risk of toxicity are advanced age and dehydration. The concurrent use of amikacin with potent diuretics (ethacrynic acid or furosemide) should be avoided since diuretics by themselves may cause ototoxicity. In addition, when administered intravenously, diuretics may enhance aminoglycoside toxicity by altering antibiotic concentrations in serum and tissue.) |
Overview
Amikacin sulfate is an antibiotic that is FDA approved for the treatment of infections due to susceptible strains of Gram-negative bacteria, including Pseudomonas species, Escherichia coli, species of indole-positive and indole-negative Proteus, Providencia species, Klebsiella-Enterobacter-Serratia species, Acinetobacter (Mima-Herellea) species, Staphylococcus and Gram-positive organisms, such as streptococcus. Clinical studies have proven efficacy in:
- Bacterial Septicemia (including neonatal sepsis)
- Severe respiratory tract infections
- Bone infections
- Joint infections
- Central nervous system infections (including meningitis)
- Skin and soft tissue infections
- Intra-abdominal infections (including peritonitis).
- Burns
- Post-operative infections (including post-vascular surgery)
- Serious complicated and recurrent urinary tract infections. There is a Black Box Warning for this drug as shown here. Common adverse reactions include neuromuscular blockade, ototoxicity, nephrotoxicity and respiratory paralysis..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Infections due to susceptible strains of Gram-negative bacteria, including Pseudomonas species, Escherichia coli, species of indole-positive and indole-negative Proteus, Providencia species, Klebsiella-Enterobacter-Serratia species, Acinetobacter (Mima-Herellea) species, Staphylococcus and Gram-positive organisms, such as streptococcus. Clinical studies have proven efficacy in:
- Bacterial Septicemia (including neonatal sepsis)
- Severe respiratory tract infections
- Bone infections
- Joint infections
- Central nervous system infections (including meningitis)
- Dosage:
- IV: 15 mg/kg/day IV divided in doses q8h.
- Intraventricular: 5mg-50 mg/day.
- Dosage:
- Skin and soft tissue infections
- Intra-abdominal infections (including peritonitis).
- Burns
- Post-operative infections (including post-vascular surgery)
- Serious complicated and recurrent urinary tract infections
Dosage
Intramuscular Administration for Patients with Normal Renal Function
The recommended dosage for adults, children and older infants with normal renal function is 15 mg/kg/day divided into 2 or 3 equal doses administered at equally divided intervals, i.e., 7.5 mg/kg q12h or 5 mg/kg q8h. Treatment of patients in the heavier weight classes should not exceed 1.5 g/day. When amikacin is indicated in newborns, it is recommended that a loading dose of 10 mg/kg be administered initially to be followed with 7.5 mg/kg every 12 hours.
The usual duration of treatment is 7 to 10 days. It is desirable to limit the duration of treatment to short-term whenever feasible. The total daily dose by all routes of administration should not exceed 15 mg/kg/day. In difficult and complicated infections where treatment beyond 10 days is considered, the use of amikacin should be reevaluated. If continued, amikacin serum levels and renal, auditory and vestibular functions should be monitored. At the recommended dosage level, uncomplicated infections due to amikacin-sensitive organisms should respond in 24 to 48 hours. If definite clinical response does not occur within 3 to 5 days, therapy should be stopped and the antibiotic susceptibility pattern of the invading organism should be rechecked. Failure of the infection to respond may be due to resistance of the organism or to the presence of septic foci requiring surgical drainage. When amikacin is indicated in uncomplicated urinary tract infections, a dose of 250 mg twice daily may be used.
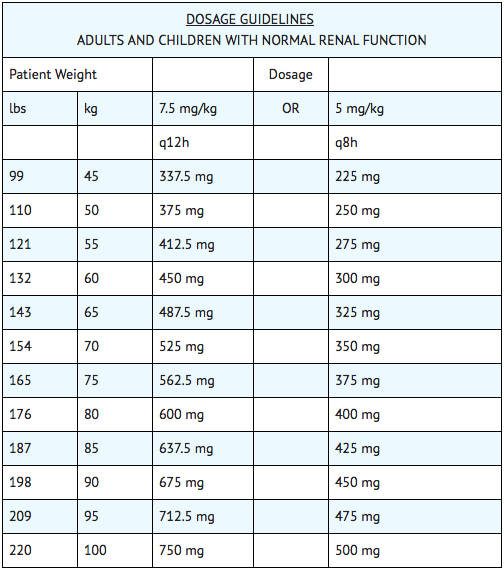
Intramuscular Administration for Patients with Impaired Renal Function
Whenever possible, serum amikacin concentrations should be monitored by appropriate assay procedures. Doses may be adjusted in patients with impaired renal function either by administering normal doses at prolonged intervals or by administering reduced doses at a fixed interval.
Both methods are based on the patient's creatinine clearance or serum creatinine values since these have been found to correlate with aminoglycoside half-lives in patients with diminished renal function. These dosage schedules must be used in conjunction with careful clinical and laboratory observations of the patient and should be modified as necessary. Neither method should be used when dialysis is being performed.
- Normal Dosage at Prolonged Intervals: If the creatinine clearance rate is not available and the patient's condition is stable, a dosage interval in hours for the normal dose can be calculated by multiplying the patient's serum creatinine by 9, e.g., if the serum creatinine concentration is 2 mg/100 mL, the recommended single dose (7.5 mg/kg) should be administered every 18 hours.
- Reduced Dosage at Fixed Time Intervals: When renal function is impaired and it is desirable to administer amikacin at a fixed time interval, dosage must be reduced. In these patients, serum amikacin concentrations should be measured to assure accurate administration of amikacin and to avoid concentrations above 35 mcg/mL. If serum assay determinations are not available and the patient's condition is stable, serum creatinine and creatinine clearance values are the most readily available indicators of the degree of renal impairment to use as a guide for dosage. First, initiate therapy by administering a normal dose, 7.5 mg/kg, as a loading dose. This loading dose is the same as the normally recommended dose which would be calculated for a patient with a normal renal function as described above. To determine the size of maintenance doses administered every 12 hours, the loading dose should be reduced in proportion to the reduction in the patient's creatinine clearance rate:

Intravenous Administrations
The individual dose, the total daily dose, and the total cumulative dose of amikacin sulfate are identical to the dose recommended for intramuscular administration. The solution for intravenous use is prepared by adding the contents of a 500 mg vial to 100 or 200 mL of sterile diluent such as 0.9% sodium chloride injection or 5% dextrose injection or any other compatible solutions listed below. The solution is administered to adults over a 30 to 60 minute period. The total daily dose should not exceed 15 mg/kg/day and may be divided into either 2 or 3 equally-divided doses at equally-divided intervals.
In pediatric patients, the amount of fluid used will depend on the amount of amikacin sulfate ordered for the patient. It should be a sufficient amount to infuse the amikacin over a 30 to 60 minute period. Infants should receive a 1 to 2 hour infusion.
Amikacin should not be physically premixed with other drugs but should be administered separately according to the recommended dose and route.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Febrile Neutropenia
- 2010 Guideline - Treatment of febrile neutropenia in cancer patients [1]
Non–Guideline-Supported Use
Bacterial Endocarditis
- Dosage: Amikacin[2] 7.5 mg/kg/12h + Cloxacillin 2 g/4h (Duration of therapy: 2 weeks).
Eye Infection
- Dosage: 25 mg subconjunctival
Febrile Neutropenia
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Bacterial Meningitis
- Dosage:
- IV: 15 mg/kg/day IV divided in doses q8h.
- Intraventricular: 5mg-50 mg/day.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Amikacin sulfate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Amikacin sulfate in pediatric patients.
Contraindications
There is limited information regarding Amikacin sulfate Contraindications in the drug label.
Warnings
|
Warning
See full prescribing information for complete Boxed Warning.
Condition Name: Patients treated with parenteral aminoglycosides should be under close clinical observation because of the potential ototoxicity and nephrotoxicity associated with their use. Safety for treatment periods which are longer than 14 days has not been established.
Neurotoxicity, manifested as vestibular and permanent bilateral auditory ototoxicity, can occur in patients with preexisting renal damage and in patients with normal renal function treated at higher doses and/or for periods longer than those recommended. The risk of aminoglycoside-induced ototoxicity is greater in patients with renal damage. High frequency deafness usually occurs first and can be detected only by audiometric testing. Vertigo may occur and may be evidence of vestibular injury. Other manifestations of neurotoxicity may include numbness, skin tingling, muscle twitching and convulsions. The risk of hearing loss due to aminoglycosides increases with the degree of exposure to either high peak or high trough serum concentrations. Patients developing cochlear damage may not have symptoms during therapy to warn them of developing eighth-nerve toxicity, and total or partial irreversible bilateral deafness may occur after the drug has been discontinued. Aminoglycoside-induced ototoxicity is usually irreversible. Aminoglycosides are potentially nephrotoxic. The risk of nephrotoxicity is greater in patients with impaired renal function and in those who receive high doses or prolonged therapy. Neuromuscular blockade and respiratory paralysis have been reported following parenteral injection, topical instillation (as in orthopedic and abdominal irrigation or in local treatment of empyema), and following oral use of aminoglycosides. The possibility of these phenomena should be considered if aminoglycosides are administered by any route, especially in patients receiving anesthetics; neuromuscular blocking agents such as tubocurarine, succinylcholine, decamethonium; or in patients receiving massive transfusions of citrate-anticoagulated blood. If blockage occurs, calcium salts may reverse these phenomena, but mechanical respiratory assistance may be necessary. Renal and eighth-nerve function should be closely monitored especially in patients with known or suspected renal impairment at the onset of therapy and also in those whose renal function is initially normal but who develop signs of renal dysfunction during therapy. Serum concentrations of amikacin should be monitored when feasible to assure adequate levels and to avoid potentially toxic levels and prolonged peak concentrations above 35 micrograms per mL. Urine should be examined for decreased specific gravity, increased excretion of proteins and the presence of cells or casts. Blood urea nitrogen, serum creatinine or creatinine clearance should be measured periodically. Serial audiograms should be obtained where feasible in patients old enough to be tested, particularly high risk patients. Evidence of ototoxicity (dizziness, vertigo, tinnitus, roaring in the ears and hearing loss) or nephrotoxicity requires discontinuation of the drug or dosage adjustment. Concurrent and/or sequential systemic, oral or topical use of other neurotoxic or nephrotoxic products, particularly bacitracin, cisplatin, amphotericin B, cephaloridine, paromomycin, viomycin, polymyxin B, colistin, vancomycin or other aminoglycosides should be avoided. Other factors that may increase risk of toxicity are advanced age and dehydration. The concurrent use of amikacin with potent diuretics (ethacrynic acid or furosemide) should be avoided since diuretics by themselves may cause ototoxicity. In addition, when administered intravenously, diuretics may enhance aminoglycoside toxicity by altering antibiotic concentrations in serum and tissue.) |
There is limited information regarding Amikacin sulfate Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Amikacin sulfate Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Amikacin sulfate Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Amikacin sulfate Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Amikacin sulfate in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Amikacin sulfate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Amikacin sulfate during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Amikacin sulfate in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Amikacin sulfate in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Amikacin sulfate in geriatric settings.
Gender
There is no FDA guidance on the use of Amikacin sulfate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Amikacin sulfate with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Amikacin sulfate in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Amikacin sulfate in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Amikacin sulfate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Amikacin sulfate in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Amikacin sulfate Administration in the drug label.
Monitoring
There is limited information regarding Amikacin sulfate Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Amikacin sulfate and IV administrations.
Overdosage
There is limited information regarding Amikacin sulfate overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Amikacin sulfate Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Amikacin sulfate Mechanism of Action in the drug label.
Structure
There is limited information regarding Amikacin sulfate Structure in the drug label.
Pharmacodynamics
There is limited information regarding Amikacin sulfate Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Amikacin sulfate Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Amikacin sulfate Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Amikacin sulfate Clinical Studies in the drug label.
How Supplied
There is limited information regarding Amikacin sulfate How Supplied in the drug label.
Storage
There is limited information regarding Amikacin sulfate Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Amikacin sulfate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Amikacin sulfate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Amikacin sulfate Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Amikacin sulfate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Amikacin sulfate Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Amikacin sulfate Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america" (PDF).
- ↑ Torres-Tortosa M, de Cueto M, Vergara A, Sánchez-Porto A, Pérez-Guzmán E, González-Serrano M; et al. (1994). "Prospective evaluation of a two-week course of intravenous antibiotics in intravenous drug addicts with infective endocarditis. Grupo de Estudio de Enfermedades Infecciosas de la Provincia de Cádiz". Eur J Clin Microbiol Infect Dis. 13 (7): 559–64. PMID 7805683.