3 beta-hydroxysteroid dehydrogenase deficiency: Difference between revisions
No edit summary |
|||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{CMG}}; {{AE}} {{Ammu}} | {{CMG}}; {{AE}} {{Ammu}} | ||
{{SI}} | |||
==Overview== | ==Overview== | ||
3β-Hydroxysteroid dehydrogenase II-deficient congenital adrenal hyperplasia (3βHSD CAH) is an uncommon form of [[congenital adrenal hyperplasia]] resulting from a defective [[gene]] for one of the key [[enzyme]]s in [[cortisol]] synthesis by the [[adrenal gland]]s. 3βHSD Congenital adrenal hyperplasia can cause salt-wasting adrenal crises in infancy. It can also cause mild [[virilization]] of genetically female infants and undervirilization of genetically male infants, making it the only form of CAH which can cause [[ambiguous genitalia]] in both genetic sexes. | 3β-Hydroxysteroid dehydrogenase II-deficient congenital adrenal hyperplasia (3βHSD CAH) is an uncommon form of [[congenital adrenal hyperplasia]] resulting from a defective [[gene]] for one of the key [[enzyme]]s in [[cortisol]] synthesis by the [[adrenal gland]]s. 3βHSD Congenital adrenal hyperplasia can cause salt-wasting adrenal crises in infancy. It can also cause mild [[virilization]] of genetically female infants and undervirilization of genetically male infants, making it the only form of CAH which can cause [[ambiguous genitalia]] in both genetic sexes. | ||
Revision as of 18:53, 7 August 2017
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Overview
3β-Hydroxysteroid dehydrogenase II-deficient congenital adrenal hyperplasia (3βHSD CAH) is an uncommon form of congenital adrenal hyperplasia resulting from a defective gene for one of the key enzymes in cortisol synthesis by the adrenal glands. 3βHSD Congenital adrenal hyperplasia can cause salt-wasting adrenal crises in infancy. It can also cause mild virilization of genetically female infants and undervirilization of genetically male infants, making it the only form of CAH which can cause ambiguous genitalia in both genetic sexes.
Severe 3β-HSD II-deficient congenital adrenal hyperplasia is uncommon, and can cause salt-wasting due to mineralocorticoid deficiency. The most distinctive aspect of sex hormone metabolism in severe deficiency is that the newborn genitalia of both sexes can be affected.
Congenital Adrenal Hyperplasia
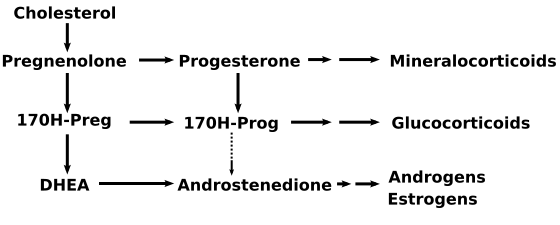
- Congenital adrenal hyperplasia refers to any of several autosomal recessive diseases resulting from defects in steps of the synthesis of cortisol from cholesterol by the adrenal glands. All of the forms of congenital adrenal hyperplasia involve the excessive or defective production of sex steroids and can pervert or impair the development of primary or secondary sex characteristics in affected infants, children, and adults. Many also involve the excessive or defective production of mineralocorticoids, which can cause hypertension or salt wasting.
- The most common type of congenital adrenal hyperplasia is due to deficiency of 21-hydroxylase and is covered in detail in the main article on congenital adrenal hyperplasia. 3β HSD CAH is one of the less common types of congenital adrenal hyperplasia due to deficiencies of other proteins and enzymes involved in cortisol synthesis.
Pathophysiology
- 3β-HSD II mediates three parallel dehydrogenase/isomerase reactions in the adrenals that convert Δ4 to Δ5 steroids: 17-Hydroxypregnenolone to 17-Hydroxyprogesterone, DHEA to androstenedione, and pregnenolone to progesterone. 3β -HSD II also mediates an alternate route of testosterone synthesis from androstenediol in the testes. 3β-HSD deficiency results in large elevations of pregnenolone, 17-hydroxypregnenolone, and DHEA.
- However, complexity arises from the presence of a second 3β-HSD (3β-HSD I) coded by a different gene, expressed in the liver and placenta, and unaffected in 3β-HSD deficient congenital adrenal hyperplasia. The presence of this second enzyme has two clinical consequences. First, 3β-HSD II can convert enough of the excess 17-hydroxypregnenolone to 17OHP to produce 17OHP levels suggestive of common 21-hydroxylase deficient congenital adrenal hyperplasia. Measurement of the other affected steroids distinguishes the two. Second, 3β-HSD II can convert enough DHEA to testosterone to moderately virilize a genetically female fetus.
Mineralocorticoid aspects of 3β-HSD Cogenital Adrenal Hyperplasia
- The mineralocorticoid aspect of severe 3β -HSD congenital adrenal hyperplasia is similar to those of 21-hydroxylase deficiency. Like other enzymes involved in early stages of both aldosterone and cortisol synthesis, the severe form of 3β -HSD deficiency can result in life-threatening salt-wasting in early infancy. Salt-wasting is managed acutely with saline and high-dose hydrocortisone, and long-term fludrocortisone.
Sex steroid aspects of 3β-HSD Cogenital Adrenal Hyperplasia
- The sex steroid consequences of severe 3β -HSD congenital adrenal hyperplasia is unique among the congenital adrenal hyperplasias: it is the only form of congenital adrenal hyperplasia that can produce ambiguity in both sexes. As with 21-hydroxylase deficient congenital adrenal hyperplasia, the degree of severity can determine the magnitude of over- or undervirilization.
- In an XX (genetically female) fetus, elevated amounts of dehydroepiandrosterone can produce moderate virilization by conversion in the liver to testosterone. Virilization of genetic females is partial, often mild, and rarely raises assignment questions. The issues surrounding corrective surgery of the virilized female genitalia are the same as for moderate 21-hydroxylase deficiency but surgery is rarely considered desirable.
- The extent to which mild 3β -HSD congenital adrenal hyperplasia can cause the early appearance of pubic hair and other aspects of hyperandrogenism in later childhood or adolescence is unsettled. Early reports about 20 years ago suggesting that mild forms of 3β -HSD congenital adrenal hyperplasia comprised significant proportions of girls with premature pubic hair or older women with hirsutism have not been confirmed and it now appears that premature pubarche in childhood and hirsutism after adolescence are not common manifestations of 3β -HSD congenital adrenal hyperplasia.
- Undervirilization of genetic males with 3β -HSD congenital adrenal hyperplasia occurs because synthesis of testosterone is impaired in both adrenals and testes. Although dehydroepiandrosterone is elevated, it is a weak androgen and too little testosterone is produced in the liver to offset the deficiency of testicular testosterone. The degree of undervirilization is more variable, from mild to severe. Management issues are those of an underutilized male with normal sensitivity to testosterone.
- If the infant boy is only mildly underutilized, the hypospadias can be surgically repaired, testes brought into the scrotum, and testosterone supplied at puberty.
- Management decisions are more difficult for a moderately or severely underutilized genetic male whose testes are in the abdomen and whose genitalia look at least as much female as male. Male sex can assign and major reconstructive surgery was done to close the midline of the perineum and move the testes into a constructed scrotum. Female sex can be assigned and the testes removed and vagina enlarged surgically. A recently advocated third choice would be to assign either sex and defer surgery to adolescence. Each approach carries its own disadvantages and risks. Children and their families are different enough that none of the courses is appropriate for all.
Diagnosis
- Like the other forms of congenital adrenal hyperplasia, suspicion of severe 3β -HSD congenital adrenal hyperplasia is usually raised by the appearance of the genitalia at birth or b the development of a salt-wasting crisis in the first month of life. The diagnosis is usually confirmed by the distinctive pattern of adrenal steroids: elevated pregnenolone, 17-hydroxypregnenolone, dehydroepiandrosterone, and renin. In clinical circumstances, this form of congenital adrenal hyperplasia has sometimes been difficult to distinguish from the more common 21-hydroxylase deficient congenital adrenal hyperplasia because of the 17OHP elevation, or from simple premature adrenarche because of the dehydroepiandrosterone elevation.
Management of 3β-HSD II-deficient Congenital Adrenal Hyperplasia after Infancy
- Some of the childhood management issues are similar those of 21-hydroxylase deficiency:
- Replacing mineralocorticoid with fludrocortisone;
- Suppressing dehydroepiandrosterone and replacing cortisol with glucocorticoid;
- Providing extra glucocorticoid for stress;
- Close monitoring and perhaps other adjunctive measures to optimize growth.
- Deciding whether surgical repair of virilized female genitalia is warranted
- However, unlike 21-hydroxylase congenital adrenal hyperplasia, children with 3β- HSD congenital adrenal hyperplasia may be unable to produce adequate amounts of testosterone (boys) or estradiol (girls) to effect normal pubertal changes. Replacement testosterone or estrogen and progesterone can be initiated at adolescence and continued throughout adult life. Fertility may be impaired by the difficulty of providing appropriate sex hormone levels in the gonads even though the basic anatomy is present.