Emtricitabine rilpivirine tenofovir clinical pharmacology
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Mohamed Moubarak, M.D. [2]
Clinical Pharmacology
Pharmacodynamics
Effects on Electrocardiogram
The effect of rilpivirine at the recommended dose of 25 mg once daily on the QTcF interval was evaluated in a randomized, placebo and active (moxifloxacin 400 mg once daily) controlled crossover study in 60 healthy adults, with 13 measurements over 24 hours at steady state. The maximum mean time-matched (95% upper confidence bound) differences in QTcF interval from placebo after baseline-correction was 2.0 (5.0) milliseconds (i.e., below the threshold of clinical concern).
When supratherapeutic doses of 75 mg once daily and 300 mg once daily of rilpivirine were studied in healthy adults, the maximum mean time-matched (95% upper confidence bound) differences in QTcF interval from placebo after baseline-correction were 10.7 (15.3) and 23.3 (28.4) milliseconds, respectively. Steady-state administration of rilpivirine 75 mg once daily and 300 mg once daily resulted in a mean steady-state Cmax approximately 2.6-fold and 6.7-fold, respectively, higher than the mean Cmax observed with the recommended 25 mg once daily dose of rilpivirine.
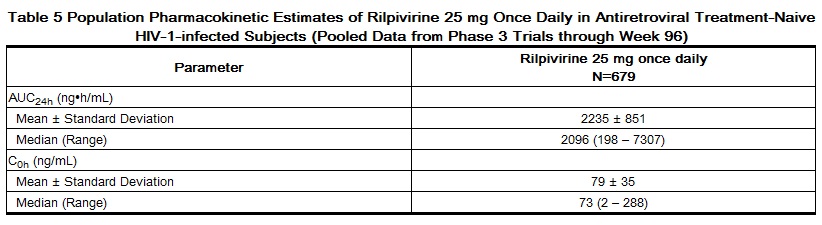
Pharmacokinetics
COMPLERA: Under fed conditions (total calorie content of the meal was approximately 400 kcal with approximately 13 grams of fat), rilpivirine, emtricitabine and tenofovir exposures were bioequivalent when comparing COMPLERA to EMTRIVA capsules (200 mg) plus Edurant tablets (25 mg) plus VIREAD tablets (300 mg) following single-dose administration to healthy subjects (N=34).
Single-dose administration of COMPLERA tablet to healthy subjects under fasted conditions provided approximately 25% higher exposure of rilpivirine compared to administration of EMTRIVA capsules (200 mg) plus Edurant tablets (25 mg) plus VIREAD tablets (300 mg), while exposures of emtricitabine and tenofovir were comparable (N=15).
Emtricitabine: Following oral administration, emtricitabine is absorbed with peak plasma concentrations occurring at 1–2 hours post-dose. Following multiple dose oral administration of EMTRIVA to 20 HIV-1-infected subjects, the mean steady-state plasma emtricitabine Cmax was 1.8 ± 0.7 µg per mL and the AUC over a 24-hour dosing interval was 10.0 ± 3.1 µg∙hr per mL. The mean steady state plasma trough concentration at 24 hours post-dose was 0.09 µg per mL. The mean absolute bioavailability of EMTRIVA capsules was 93%. Less than 4% of emtricitabine binds to human plasma proteins in vitro over the range of 0.02 to 200 µg per mL. Following administration of radiolabelled emtricitabine, approximately 86% is recovered in the urine, approximately 14% in the feces and 13% is recovered as metabolites in the urine. The metabolites of emtricitabine include 3′-sulfoxide diastereomers (approximately 9% of the dose) and the glucuronic acid conjugate (approximately 4% of the dose). Emtricitabine is eliminated by a combination of glomerular filtration and active tubular secretion with a renal clearance in adults with creatinine clearance >80 mL per minute of 213 ± 89 mL per minute (mean ± SD). The plasma emtricitabine half-life is approximately 10 hours.
Rilpivirine: The pharmacokinetic properties of rilpivirine have been evaluated in adult healthy subjects and in adult antiretroviral treatment-naive HIV-1-infected subjects. Exposure to rilpivirine was generally lower in HIV-1-infected subjects than in healthy subjects. After oral administration, the Cmax of rilpivirine is achieved within 4–5 hours. The absolute bioavailability of rilpivirine is unknown.
Rilpivirine is approximately 99.7% bound to plasma proteins in vitro, primarily to albumin. In vitro experiments indicate that rilpivirine primarily undergoes oxidative metabolism by the cytochrome CYP3A system. The terminal elimination half-life of rilpivirine is approximately 50 hours. After single dose oral administration of 14C-rilpivirine, on average 85% and 6.1% of the radioactivity could be retrieved in feces and urine, respectively. In feces, unchanged rilpivirine accounted for on average 25% of the administered dose. Only trace amounts of unchanged rilpivirine (less than 1% of dose) were detected in urine.
Tenofovir Disoproxil Fumarate: Following oral administration of a single 300 mg dose of VIREAD to HIV-1-infected subjects in the fasted state, Cmax was achieved in one hour. Cmax and AUC values were 0.30 ± 0.09 µg per mL and 2.29 ± 0.69 µg∙hr per mL, respectively. The oral bioavailability of tenofovir from VIREAD in fasted subjects is approximately 25%. Less than 0.7% of tenofovir binds to human plasma proteins in vitro over the range of 0.01 to 25 µg per mL. Approximately 70–80% of the intravenous dose of tenofovir is recovered as unchanged drug in the urine within 72 hours of dosing. Tenofovir is eliminated by a combination of glomerular filtration and active tubular secretion with a renal clearance in adults with creatinine clearance >80 mL per minute of 243.5 ± 33.3 mL per minute (mean ± SD). Following a single oral dose, the terminal elimination half-life of tenofovir is approximately 17 hours.
Effects of Food on Oral Absorption
The food effect trial for COMPLERA evaluated two types of meals. The trial defined a meal with 390 kcal containing 12 g fat as a light meal, and a meal with 540 kcal containing 21 g fat as a standard meal. Relative to fasting conditions, the administration of COMPLERA to healthy adult subjects with both types of meals resulted in increased exposures of rilpivirine and tenofovir. The Cmax and AUC of rilpivirine increased 34% and 9% with a light meal, while increasing 26% and 16% with a standard meal, respectively. The Cmax and AUC of tenofovir increased 12% and 28% with a light meal, while increasing 32% and 38% with a standard meal, respectively. Emtricitabine exposures were not affected by food.
The effects on rilpivirine, emtricitabine and tenofovir exposure when COMPLERA is administered with a high fat meal were not evaluated.
COMPLERA should be taken with food.
Special Populations
Race
Emtricitabine: No pharmacokinetic differences due to race have been identified following the administration of EMTRIVA.
Rilpivirine: Population pharmacokinetic analysis of rilpivirine in HIV-1-infected subjects indicated that race had no clinically relevant effect on the exposure to rilpivirine.
Tenofovir Disoproxil Fumarate: There were insufficient numbers from racial and ethnic groups other than Caucasian to adequately determine potential pharmacokinetic differences among these populations following the administration of VIREAD.
Gender
No clinically relevant pharmacokinetic differences have been observed between men and women for emtricitabine, rilpivirine, and tenofovir DF.
Pediatric Patients
Emtricitabine has been studied in pediatric subjects from 3 months to 17 years of age. Tenofovir DF has been studied in adolescent subjects (12 to less than 18 years of age). The pharmacokinetics of rilpivirine in pediatric subjects have not been established.
Geriatric Patients
Pharmacokinetics of emtricitabine, rilpivirine and tenofovir have not been fully evaluated in the elderly (65 years of age and older) [See Use in Specific Populations (8.5)].
Patients with Renal Impairment
Emtricitabine and Tenofovir Disoproxil Fumarate: The pharmacokinetics of emtricitabine and tenofovir DF are altered in subjects with renal impairment. In subjects with creatinine clearance below 50 mL per minute or with end stage renal disease requiring dialysis, Cmax, and AUC of emtricitabine and tenofovir were increased [See Warnings and Precautions (5.3) and Use in Specific Populations (8.6)].
Rilpivirine: Population pharmacokinetic analysis indicated that rilpivirine exposure was similar in HIV-1-infected subjects with mild renal impairment relative to HIV-1-infected subjects with normal renal function. There is limited or no information regarding the pharmacokinetics of rilpivirine in patients with moderate or severe renal impairment or in patients with end-stage renal disease, and rilpivirine concentrations may be increased due to alteration of drug absorption, distribution, and metabolism secondary to renal dysfunction [See Use in Specific Populations (8.6)].
Patients with Hepatic Impairment
Emtricitabine: The pharmacokinetics of emtricitabine have not been studied in subjects with hepatic impairment; however, emtricitabine is not significantly metabolized by liver enzymes, so the impact of liver impairment should be limited.
Rilpivirine: Rilpivirine is primarily metabolized and eliminated by the liver. In a study comparing 8 subjects with mild hepatic impairment (Child-Pugh score A) to 8 matched controls and 8 subjects with moderate hepatic impairment (Child-Pugh score B) to 8 matched controls, the multiple dose exposure of rilpivirine was 47% higher in subjects with mild hepatic impairment and 5% higher in subjects with moderate hepatic impairment [See Use in Specific Populations (8.7)].
Tenofovir Disoproxil Fumarate: The pharmacokinetics of tenofovir following a 300 mg dose of VIREAD have been studied in non-HIV-infected subjects with moderate to severe hepatic impairment. There were no substantial alterations in tenofovir pharmacokinetics in subjects with hepatic impairment compared with unimpaired subjects.
Hepatitis B and/or Hepatitis C Virus Coinfection
Pharmacokinetics of emtricitabine and tenofovir DF have not been fully evaluated in hepatitis B and/or C virus-coinfected patients. Population pharmacokinetic analysis indicated that hepatitis B and/or C virus coinfection had no clinically relevant effect on the exposure to rilpivirine.[1]
References
Adapted from the FDA Package Insert.