Emtricitabine rilpivirine tenofovir description
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Mohamed Moubarak, M.D. [2]
Description
COMPLERA is a fixed-dose combination tablet containing emtricitabine, rilpivirine hydrochloride, and tenofovir DF. EMTRIVA is the brand name for emtricitabine, a synthetic nucleoside analog of cytidine. Edurant is the brand name for rilpivirine, a non-nucleoside reverse transcriptase inhibitor. VIREAD is the brand name for tenofovir DF, which is converted in vivo to tenofovir, an acyclic nucleoside phosphonate (nucleotide) analog of adenosine 5′-monophosphate. VIREAD and EMTRIVA are the components of TRUVADA.
COMPLERA tablets are for oral administration. Each tablet contains 200 mg of emtricitabine, 27.5 mg of rilpivirine hydrochloride (equivalent to 25 mg of rilpivirine), and 300 mg of tenofovir DF (equivalent to 245 mg of tenofovir disoproxil) as active ingredients. The tablets include the following inactive ingredients: pregelatinized starch, lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, magnesium stearate, povidone, polysorbate 20. The tablets are film-coated with a coating material containing polyethylene glycol, hypromellose, lactose monohydrate, triacetin, titanium dioxide, iron oxide red, FD&C Blue #2 aluminum lake, FD&C Yellow #6 aluminum lake.
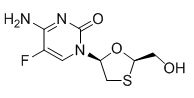
Emtricitabine: The chemical name of emtricitabine is 5-fluoro-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine. Emtricitabine is the (–) enantiomer of a thio analog of cytidine, which differs from other cytidine analogs in that it has a fluorine in the 5-position.
It has a molecular formula of C8H10FN3O3S and a molecular weight of 247.24. It has the following structural formula:
Emtricitabine is a white to off-white crystalline powder with a solubility of approximately 112 mg per mL in water at 25 °C.
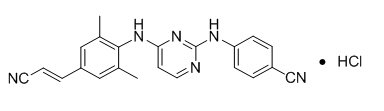
Rilpivirine: Rilpivirine is available as the hydrochloride salt. The chemical name for rilpivirine hydrochloride is 4-[[4-[[4-[(E)-2-cyanoethenyl]-2,6-dimethylphenyl]amino]-2-pyrimidinyl]amino]benzonitrile monohydrochloride. Its molecular formula is C22H18N6 • HCl and its molecular weight is 402.88. Rilpivirine hydrochloride has the following structural formula:
Rilpivirine hydrochloride is a white to almost white powder. Rilpivirine hydrochloride is practically insoluble in water over a wide pH range.
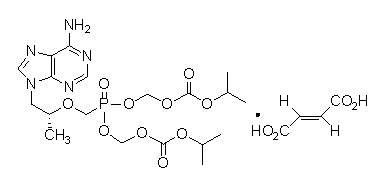
Tenofovir Disoproxil Fumarate: Tenofovir DF is a fumaric acid salt of the bis-isopropoxycarbonyloxymethyl ester derivative of tenofovir. The chemical name of tenofovir DF is 9-[(R)-2 [[bis[[(isopropoxycarbonyl)oxy]- methoxy]phosphinyl]methoxy]propyl]adenine fumarate (1:1). It has a molecular formula of C19H30N5O10P • C4H4O4 and a molecular weight of 635.52. It has the following structural formula:
Tenofovir DF is a white to off-white crystalline powder with a solubility of 13.4 mg per mL in water at 25 °C. All dosages are expressed in terms of tenofovir DF except where otherwise noted.[1]
References
Adapted from the FDA Package Insert.