Pravastatin detailed information
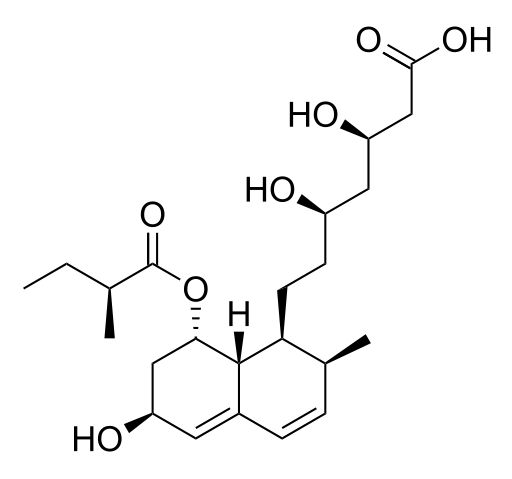 | |
| Clinical data | |
|---|---|
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | 50% |
| Elimination half-life | 77 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C23H36O7 |
| Molar mass | 424.528 g/mol |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [2] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
For patient information, click here
Overview
In medicine and pharmacology, pravastatin (Pravachol® or Selektine®) is a member of the drug class of statins, used for lowering cholesterol and preventing cardiovascular disease.
Pravastatin was identified originally in a bacterium called Nocardia autotrophica by researchers of the Sankyo Pharma Inc. It is presently being marketed outside Japan by the pharmaceutical company Bristol-Myers Squibb.
In 2003, Bristol-Myers Squibb announced in a magazine advertisement that pravastatin is no longer the only statin approved by the FDA proven to reduce the risk of cardiovascular disease, and that rosuvastatin, another statin, is approved to reduce this risk.
The U.S. Food and Drug Administration approved a generic version of Pravastatin for sale in the United States for the first time on April 24, 2006. Generic Pravastatin Sodium Tablets (10mg, 20mg and 40mg) are manufactured by TEVA Pharmaceuticals in Kfar Sava, Israel. FDA Press Release
In 2005, Pravachol was the 22nd highest-selling brand-name drug in the United States. FDA Press Release
External links
Template:Statins Template:SIB hu:Pravasztatin nl:Pravastatine th:ปราวาสแตติน
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Statins
- Bristol-Myers Squibb
- Drugs
- Cardiology