Huntington's disease pathophysiology
| https://https://www.youtube.com/watch?v=IuSaXiRVqg0%7C350}} |
|
Huntington's disease Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Huntington's disease pathophysiology On the Web |
|
American Roentgen Ray Society Images of Huntington's disease pathophysiology |
|
Risk calculators and risk factors for Huntington's disease pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Syed Ahsan Hussain, M.D.[2]
Overview
Pathophysiology
Genetics
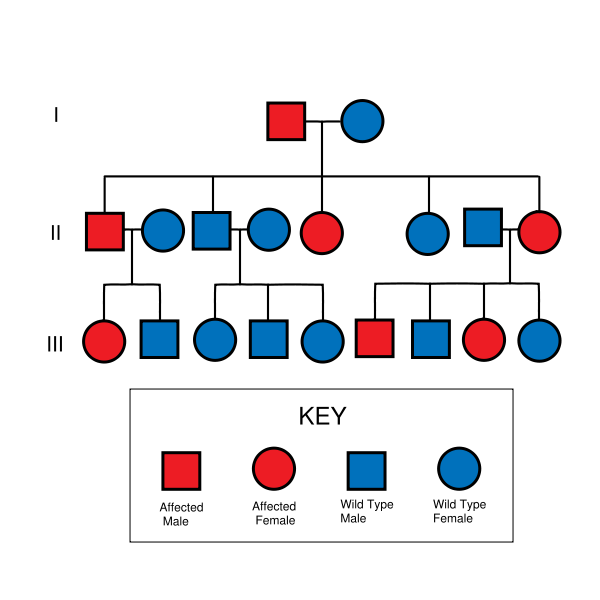
- Huntington's disease is autosomal dominant, needing only one affected allele from either parent to inherit the disease. Although this generally means there is a one in two chance of inheriting the disorder from an affected parent, the inheritance of HD and other trinucleotide repeat disorders is more complex.
- The gene involved in Huntington's disease, called the HD gene or Interesting Transcript 15 (IT15), is located on the short arm of chromosome 4 (4p16.3). The end of the HD gene has a sequence of three DNAbases,cytosine-adenine-guanine (CAG), that is repeated multiple times (i.e. ...CAGCAGCAG...); this is called atrinucleotide repeat.
- The expansion of trinucleotide repeats produces an altered form of the Htt protein, mutant Huntingtin (mHtt), which results in neuronal cell death in select areas of the brain.
- CAG is the codon for the amino acid glutamine, thus a CAG repeat may be termed a polyglutamine (polyQ) expansion. A sequence of fewer than 36 glutamine amino acid residues is the normal form, producing a 348 kDa cytoplasmic protein called huntingtin (Htt). A sequence of 40 or more CAG repeats produces a mutated form of Htt, mHtt. The greater the number of CAG repeats, the earlier the onset of symptoms.[1]
- In genetically altered "knockin" mice, the mutant CAG repeat portion of the gene (which codes for the N-terminal end of mHtt) is all that is needed to cause disease.[2]
- Aggregates of mHtt are present in the brains of both HD patients[3] and HD mice,[4] specifically in striatal neurons.[5] These aggregates consist mainly of the amino terminal end of mHtt (CAG repeat), and are found in both the cytoplasm and nucleus of neurons.[6] The presence of these aggregates however does not correlate with cell death.[7] Thus mHtt acts in the nucleus but does not causeapoptosis through aggregation.[8]
- The exact mechanism by which mHtt causes or contributes towards neuronal cell death and HD symptoms remains unclear. Research exploring the actions of Htt and mHtt have shed light on the subject.
- Paternal inheritance tends to increase the number of repeats.[9]
- Because of the progressive increase in length of the repeats, the disease tends to increase in severity and have an earlier onset in successive generations. This is known as anticipation.
- De novo mutations are rare.
- Homozygous individuals generally do not show an earlier onset of disease, but may have an increased rate of decline.
- Like all proteins, Htt is translated, performs an action, and is finally degraded. Both Htt and mHtt are cleaved (the first step in degradation) by Caspase-3, which removes the (amino end) N-terminal.[10] Caspase-2 then further breaks down the amino terminal fragment of Htt, but cannot act upon mHtt.[11] The mHtt amino fragments are thus able to affect gene expression in polyQ dependent transcription.[12] Specifically, mHtt binds with TAFII130, a coactivator to CREB dependent transcription.[13] The mHtt N-fragments also interact with SP1, thereby preventing it from binding to DNA.[14] Thus mHtt alters the normal functioning of these proteins.
- Mutant Huntingtin also downregulates brain-derived neurotropic factor (BDNF) which protects striatal neurons.[15] This loss of BDNF may contribute to striatal cell death, which does not follow apoptotic pathways as the neurons appear to die of starvation.[16]
- Huntingtin appears to be involved in vesicle trafficking as it interacts with HIT1, a clathrin binding protein, to mediateendocytosis.[17][18]
- In the June 16, 2006 issue of Cell, scientists at the University of British Columbia (UBC) andMerck Labs presented findings that the neurodegeneration caused by mHtt is related to the caspase-6enzyme cleaving the Htt protein. Transgenic mice that have caspase-6 resistant Htt did not show effects of HD.[19] The researchers found "substantial support for the hypothesis that cleavage at the caspase-6 site in mHtt represents a crucial rate-limiting event in the pathogenesis of HD.... Our study highlights the importance of preventing cleavage of Htt at this site and also reinforces the importance of modulating excitotoxicity as a potential therapeutic approach for HD." In essence, scientists have managed to prevent the appearance of HD in genetically modified mice. Dr. Marian DiFiglia, a world-renowned HD researcher and neurobiologist at Harvard University, called this find "very important" and "extremely intriguing".[20]
Gross Pathology
HD affects the whole brain, but certain areas are more vulnerable than others. The most prominent early effects are in a part of the basal ganglia called the neostriatum, which is composed of the caudate nucleus and putamen.Other areas affected include the substantia nigra, layers 3, 5 and 6 of the cerebral cortex, the hippocampus, purkinje cells in the cerebellum, lateral tuberal nuclei of the hypothalamus and parts of the thalamus. These areas are affected according to their structure and the types of neurons they contain, reducing in size as they lose cells.Striatal spiny neurons are the most vulnerable, particularly ones with projections towards the external globus pallidus, with interneurons and spiny cells projecting to the internal pallidum being less affected.[21] HD also causes an abnormal increase in astrocytes and activation of the brain's immune cells, microglia.[22]
The basal ganglia—the part of the brain most prominently affected in early HD—play a key role in movement and behavior control. Their functions are not fully understood, but current theories propose that they are part of the cognitive executive system and the motor circuit.[23] The basal ganglia ordinarily inhibit a large number of circuits that generate specific movements. To initiate a particular movement, the cerebral cortex sends a signal to the basal ganglia that causes the inhibition to be released. Damage to the basal ganglia can cause the release or reinstatement of the inhibitions to be erratic and uncontrolled, which results in an awkward start to motion or motions to be unintentionally initiated, or a motion to be halted before, or beyond, its intended completion. The accumulating damage to this area causes the characteristic erratic movements associated with HD.[23]
References
- ↑ Kieburtz K, MacDonald M, Shih C, Feigin A, Steinberg K, Bordwell K, Zimmerman C, Srinidhi J, Sotack J, Gusella J, et al. Trinucleotide Repeat Length and Progression of Illness in Huntington's Disease. J Med Genet. 1994; 31:872
- ↑ Murphy K, Carter R, Lione L, Mangiarini L, Mahal A, Bates G, Dunnett S, and Morton J. Abnormal Synaptic Plasticity and Impaired Spatiail Cognition in Mice Transgenic for Exon 1 of the Human Huntington’s Disease Mutation. Journal of Neuroscience 2000; 20:5115
- ↑ Difiglia M, Sapp E, Chase E, Davies K. et al. Aggregation of Huntingtin in Neuronal Intranuclear Inclusions and Dystrophic Neurites in Brain. Science 1997
- ↑ Davies S, Turmaine M, Cozens B, Difiglia M, Sharp A, Ross C, Scherzinger E, Wanker E, Mangiarini L, and Bates G. Formation of Neuronal Intranuclear Inclusions Underlies the Neurological Dysfunction in Mice Transgenic for the HD Mutation. Cell 1997; 90:537
- ↑ Li H, Li S, Johnston H, Shelbourne P, and Li X. Amino-terminal Fragments of Mutant Huntingtin Show Selective Accumulation in Striatal Neurons and Synaptic Toxicity. Nature Genetics 2000; 25:385
- ↑ Cooper JK, Schilling G, Peters MF, Herring WJ, Sharp AH, Kaminsky Z, Masone J, Khan FA, Delanoy M, Borchelt DR, Dawson VL, Dawson TM, Ross CA. Truncated N-terminal Fragments of Huntingtin with Expanded Glutamine Repeats Form Nuclear and Cytoplasmic Aggregates in Cell Culture. Hum Mol Genet. 1998; 7:783
- ↑ F. R. Fusco, Q. Chen, W. J. Lamoreaux, G. Figueredo-Cardenas, Y. Jiao, J. A. Coffman, D. J. Surmeier, M. G. Honig, L. R. Carlock, and A. Reiner. Cellular Localization of Huntingtin in Striatal and Cortical Neurons in Rats: Lack of Correlation with Neuronal Vulnerability in Huntington's Disease. J. Neurosci 1999; 19: 1189
- ↑ Saudou F, Finkbeiner S, Devys, and Greenberg M. Huntingtin Acts in the Nucleus to Induce Apoptosis but Death Does Not Correlate with the Formation of Intranuclear Inclusions. Cell. 1998; 95:55
- ↑ RM Ridley, CD Frith, TJ Crow and PM Conneally (1988). "Anticipation in Huntington's disease is inherited through the male line but may originate in the female". Journal of Medical Genetics. 25: 589–595.
- ↑ Kim YJ, Yi Y, Sapp E, Wang Y, Cuiffo B, Kegel KB, Qin ZH, Aronin N, DiFiglia M. Caspase 3-cleaved N-terminal Fragments of Wild-type and Mutant Huntingtin are Present in Normal and Huntington's Disease Brains, Associate with Membranes, and Undergo calpain-dependent proteolysis. Proc Natl Acad Sci U S A. 2001; 98:12784
- ↑ Hermel E, Gafni J, Propp SS, Leavitt BR, Wellington CL, Young JE, Hackam AS, Logvinova AV, Peel AL, Chen SF, Hook V, Singaraja R, Krajewski S, Goldsmith PC, Ellerby HM, Hayden MR, Bredesen DE, Ellerby LM. Specific Caspase Interactions and Amplification are Involved in Selective Neuronal Vulnerability in Huntington's Disease. Cell Death Differ 2004; 11:424
- ↑ Freiman R, and Tjian R. A Glutamine-Rich Trail Leads to Transcription Factors. Science 2002; 296:2149
- ↑ Bae B.I, Xu H, Igarashi S, Fujimuro M, Agrawal N, Taya Y, Hayward S.D, Moran T.H, Montell C, Ross C.A, Snyder S.H, and Sawa A. p53 Mediates Cellular Dysfunction and Behavioral Abnormalities in Huntington's Disease. Neuron 2005; 47:29
- ↑ Dunah A, Jeong H, Griffin A, Kim Y, Standeart D, Hersch S, Mouradian M, Young A, Tanese N, and Krainc D. Sp1 and TAFII130 Transcriptional Activity Disrupted in Early Huntington’s Disease. Science 2002; 296: 2238
- ↑ Canals J, Pineda J, Torres-Peraza J, Bosch M, Martin-Ibanez R, Munoz M, Mengod G, Ernfors P, and Alberch J. Brain Derived Neurotrophic Factor Regulates the Onset and Severity of Motor Dysfunction Associated with Enkephalinergic Neuronal Degeneration in Huntington’s Disease. Neurobiology of Disease 2004; 24:7727
- ↑ Sawa A, Nagata E, Sutcliffe S, Dulloor P, Cascio MB, Ozeki Y, Roy S, Ross CA, Snyder SH. Huntingtin is Cleaved by Caspases in the Cytoplasm and Translocated to the Nucleus via Perinuclear Sites in Huntington's Disease Patient Lymphoblasts. Neurobiol Dis 2005
- ↑ Velier J, Kim M, Schwarz C, Kim T.W, Sapp E, Chase K, Aronin N, DiFiglia M. Wild-Type and Mutant Huntingtins Function in Vesicle Trafficking in the Secretory and Endocytic Pathways. Experimental Neurology 1998; 152:34
- ↑ Waelter S, Scherzinger E, Hasenbank R, Nordhoff E, Lurz R, Goehler H, Gauss C, Sathasivam K, Bates G, Lehrach H, and Wanker E. The Huntingtin Interacting Protein HIP1 is a Clathrin and α–adaptin-binding Protein Involved in Receptor Mediated Endocytosis. Human Molecular Genetics 2001; 10:1807
- ↑ Graham, RK, Y Deng, EJ Slow, B Haigh, N Bissada, G Lu, J Pearson, J Shehadeh, L Bertram, Z Murphy, SC Warby, CN Doty, S Roy, CL Wellington, BR Leavitt, LA Raymond, DW Nicholson, MR Hayden (2006-06-16). "Cleavage at the Caspase-6 site is required for neuronal dysfunction and degeneration due to mutant Huntingtin". Cell. 125: 1179–1191.
- ↑ S. Ubelacker. "Canadian Researchers cure Huntington's disease in mice". Retrieved 2006-07-16.
- ↑ Purves D, Augustine GA, Fitzpatrick D, Hall W, LaMantia A-S, McNamara JO, Williams SM (2001). "Modulation of Movement by the Basal Ganglia – Circuits within the Basal Ganglia System". In Purves D. Neuroscience (2nd ed.). Sunderland, MA: Sinauer Associates. ISBN 0-87893-742-0. Retrieved 2009-04-01.
- ↑ Lobsiger CS, Cleveland DW (2007). "Glial cells as intrinsic components of non-cell autonomous neurodegenerative disease". Nat. Neurosci. 10 (11): 1355–60. doi:10.1038/nn1988. PMC 3110080. PMID 17965655.
- ↑ 23.0 23.1 Crossman AR (2000). "Functional anatomy of movement disorders" (PDF). J. Anat. 196 (4): 519–25. doi:10.1046/j.1469-7580.2000.19640519.x. PMC 1468094. PMID 10923984.