Brucellosis pathophysiology
|
Brucellosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Brucellosis pathophysiology On the Web |
|
American Roentgen Ray Society Images of Brucellosis pathophysiology |
|
Risk calculators and risk factors for Brucellosis pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Raviteja Guddeti, M.B.B.S. [2] Danitza Lukac
Pathophysiology
Transmission

- Brucella spp. are primarily passed among animals, and they cause disease in many different vertebrates.
- Various Brucella species affect sheep, goats, cattle, deer, elk, pigs, dogs, american bishop and several other animals.
- Humans are generally infected in one of three ways:
- Eating undercooked meat or consuming unpasteurized/raw dairy products
- The most common way to be infected is by eating or drinking unpasteurized/raw dairy products.
- When sheep, goats, cows, or camels are infected, their milk becomes contaminated with the bacteria.
- If the milk from infected animals is not pasteurized, the infection will be transmitted to people who consume the milk and/or cheese products.
- Breathing in the bacteria that cause brucellosis (inhalation)
- This risk is generally greater for people in laboratories that work with the bacteria.
- Slaughterhouse and meat-packing employees have also been known to be exposed to the bacteria and ultimately become infected.
- Bacteria entering the body through skin wounds or mucous membranes
- Bacteria can also enter wounds in the skin/mucous membranes through contact with infected animals.
- This poses a problem for workers who have close contact with animals or animal excretions (newborn animals, fetuses, and excretions that may result from birth).
- Such workers may include:
- Slaughterhouse workers
- Meat-packing plant employees
- Veterinarians
- Eating undercooked meat or consuming unpasteurized/raw dairy products
- Person-to-person spread of brucellosis is extremely rare.
- Infected mothers who are breast-feeding may transmit the infection to their infants.
- Sexual transmission has been rarely reported.
- While uncommon, transmission may also occur via tissue transplantation or blood transfusions.[1]
- Liver:
Pathogenesis
- Virulent Brucella organisms can infect both nonphagocytic and phagocytic cells.
- When Brucella enters the body white blood cells (WBC) fagocitate the pathogen, particularly neutrophils and macrophages.
- WBC transports the pathogen via hematological and lymphatic routes to different organs, particulary of the reticuloendothelial system (RES).
- They multiple themselves within the vacuoles of the phagocytes without being destructed.
- Different Brucella species are classified as smooth and rough lipopolysaccharide phenotypes.
- Smooth lipopolysaccharides (S-LPS):
- B. abortus, B. melitensis, B. suis and B. neotoma
- S-LPS are more virulent than R-LPS
- S-LPS survive much more effectively than nonsmooth ones
- Rough lipopolysaccharides (R-LPS):
- B. ovis and B. canis
- Smooth lipopolysaccharides (S-LPS):
- In polymorphonuclear or mononuclear phagocytic cells, Brucella spp. uses a number of mechanisms for avoiding or suppressing bactericidal responses:
- Lipopolysaccharide and outer membrane proteins probably play a substantial role in intracellular survival.
- This may be due to the mannose and integrins receptors.
- Brucella stays within the cells because it inhibits cellular mechanisms of programmed cell death (apoptosis).
- The survival of Brucella within the cells has been associated with:
- Synthesis of antioxidant enzymes
- Production of guanosine 5 monophosphate (GMP)
- GMP inhibits: phagolysosome fusion, degranulation and activation of the myelo-peroxidase-halide system, and production of tumor necrosis factor.
- Synthesis of proteins of molecular weight 17, 24, 28, 60, and 62 kDa.
- The 24 kDa protein is acid-induced, and its production correlates with bacterial survival under acidic conditions (<pH4).
- The 17 and 28 kDa proteins are apparently specifically induced by macrophages and correlated with intracellular survival.
- Lipopolysaccharide and outer membrane proteins probably play a substantial role in intracellular survival.
- The elimination of virulent Brucella depends on activated macrophages and hence requires development of Th1 type cell-mediated responses to protein antigens.
- High iron concentrations promote the killing of Brucella, probably by favoring production of hydroxylamine and hydroxyl radical.
- The mechanisms of pathogenesis of Brucella infection in its natural host species and in humans are still not completely understood, and further studies are needed.[2][3]
Microscopic Pathology
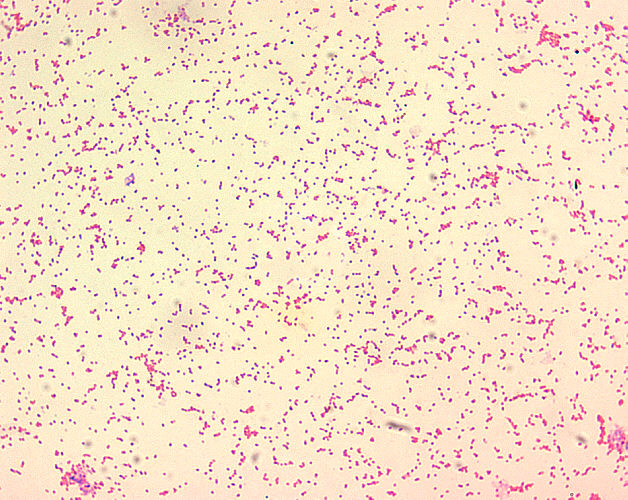
- Brucella spp. are gram-negative in their staining morphology.
- Brucella spp. are poorly staining, small gram-negative coccobacilli (0.5-0.7 x 0.6-1.5 µm).
- Brucella spp. are seen mostly as single cells and appearing like “fine sand”.[4]
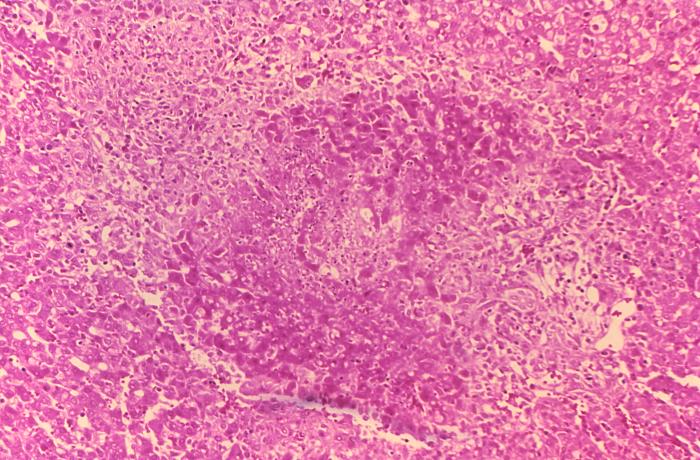
- On microscopic histopathological analysis of the liver, common findings are:
- Granulomas with centrilobular necrosis or focal necrosis and parenchyma destruction.[5]
Reference
- ↑ Brucellosis. CDC. http://www.cdc.gov/brucellosis/transmission/index.html. Accessed on January 29, 2016
- ↑ Corbel MJ (1997). "Brucellosis: an overview". Emerg Infect Dis. 3 (2): 213–21. doi:10.3201/eid0302.970219. PMC 2627605. PMID 9204307.
- ↑ Brucelosis. Wikipedia. https://es.wikipedia.org/wiki/Brucelosis. Accessed on February 2, 2016
- ↑ Brucellosis. Wikipedia. https://en.wikipedia.org/wiki/Brucellosis. Accessed on January 29, 2016
- ↑ Hunt A, Bothwell P. Histological findings in human brucellosis. J Clin Pathol. 1967; 20: 267-272