Acute myeloid leukemia pathophysiology
|
Acute myeloid leukemia Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Acute myeloid leukemia pathophysiology On the Web |
|
American Roentgen Ray Society Images of Acute myeloid leukemia pathophysiology |
|
Risk calculators and risk factors for Acute myeloid leukemia pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Raviteja Guddeti, M.B.B.S. [2] Carlos A Lopez, M.D. [3]
Overview
Acute myeloid leukemia arises from myeloblasts, which are hematologic white cells that are normally involved in hematopoiesis. Genetic translocations involved in the pathogenesis of acute myeloid leukemia include translocations between chromosome 8 and 21 t(8;21) and translocations between chromosome 15 and 17 t(15;17). Inversions involved in the pathogenesis of acute myeloid leukemia include inversions in chromosome 16 inv(16).
Pathophysiology
The malignant cell in acute myeloid leukemia is the myeloblast. In normal hematopoiesis, the myeloblast is an immature precursor of myeloid white blood cells; a normal myeloblast will gradually mature into a mature white blood cell. However, in acute myeloid leukemia a single myeloblast accumulates genetic changes which "freeze" the cell in its immature state and prevent differentiation.[1] Such a mutation alone does not cause leukemia; however, when such a "differentiation arrest" is combined with other mutations which disrupt genes controlling proliferation, the result is the uncontrolled growth of an immature clone of cells, leading to the clinical entity of acute myeloid leukemia.[2]
Much of the diversity and heterogeneity of acute myeloid leukemia stems from the fact that leukemic transformation can occur at a number of different steps along the differentiation pathway.[3] Modern classification schemes for acute myeloid leukemia recognize that the characteristics and behavior of the leukemic cell (and the leukemia) may depend on the stage at which differentiation was halted.
Specific cytogenetic abnormalities can be found in many patients with acute myeloid leukemia; the types of chromosomal abnormalities often have prognostic significance.[4] The chromosomal translocations encode abnormal fusion proteins, usually transcription factors whose altered properties may cause the "differentiation arrest."[5] For example, in acute promyelocytic leukemia, the t(15;17) translocation produces a PML-RARα fusion protein which binds to the retinoic acid receptor element in the promoters of several myeloid-specific genes and inhibits myeloid differentiation.[6]
The clinical signs and symptoms of acute myeloid leukemia result from the fact that, as the leukemic clone of cells grows, it tends to displace or interfere with the development of normal blood cells in the bone marrow.[7] This leads to neutropenia, anemia, and thrombocytopenia. The symptoms of acute myeloid leukemia are in turn often due to the low numbers of these normal blood elements. In rare cases, patients can develop a chloroma, or solid tumor of leukemic cells outside the bone marrow, which can cause various symptoms depending on its location.
Microscopic Pathology
(Images shown below are courtesy of Melih Aktan MD., Istanbul Medical Faculty - Turkey, and Kyoto University - Japan)
-
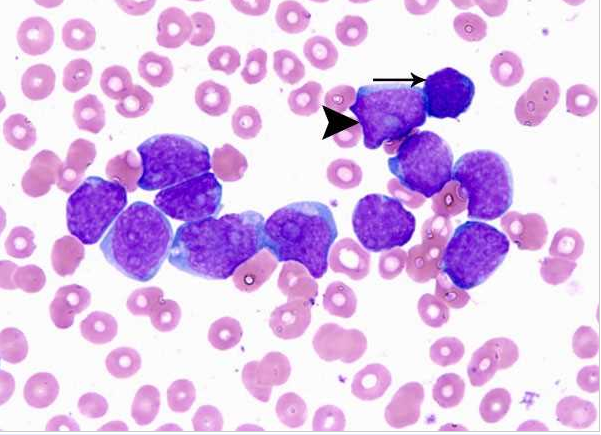
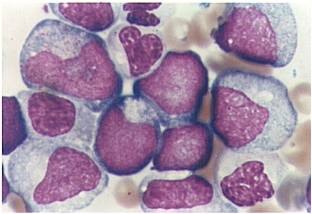
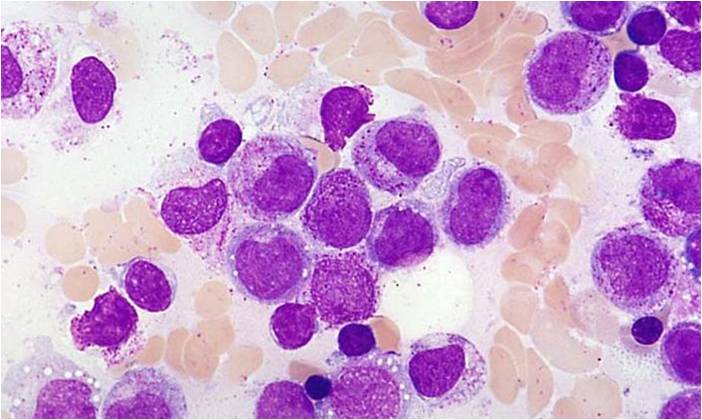
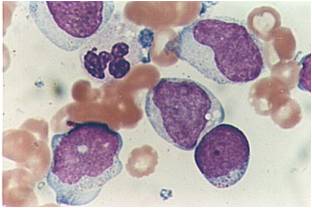
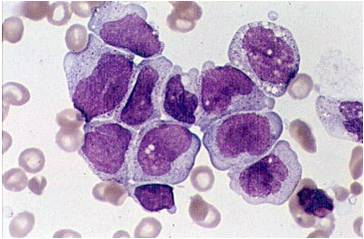
Acute myeloid leukemia-M0 - lack of obvious myeloid differentiation by routine histologic examination and presence of myeloperoxidase in <3% of blasts. Morphologically, blasts are small to large with no granules or Auer rods.
-
Acute myeloid leukemia-M1 - presence of more than 90% myeloblasts in blood.
-
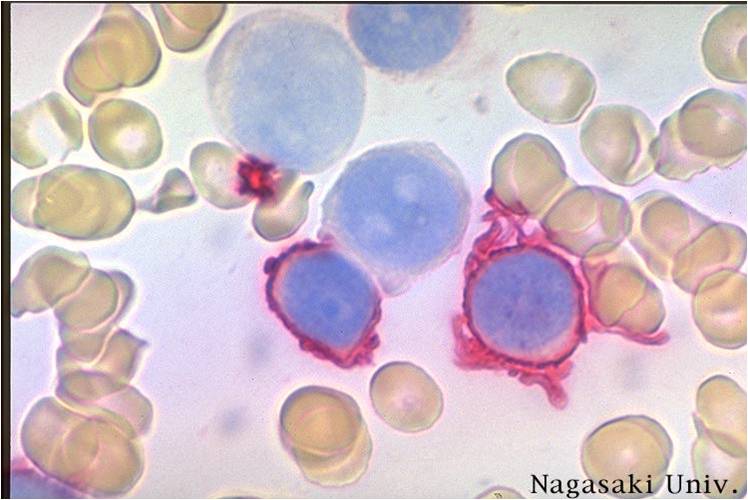
Acute myeloid leukemia-M1 peroxidase.
-
Acute myeloid leukemia-M1 - more cytoplasm than M0, but still no granules.
-
Acute myeloid leukemia-M1.
-
Acute myeloid leukemia-M1.
-
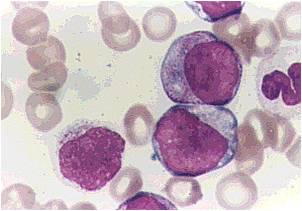
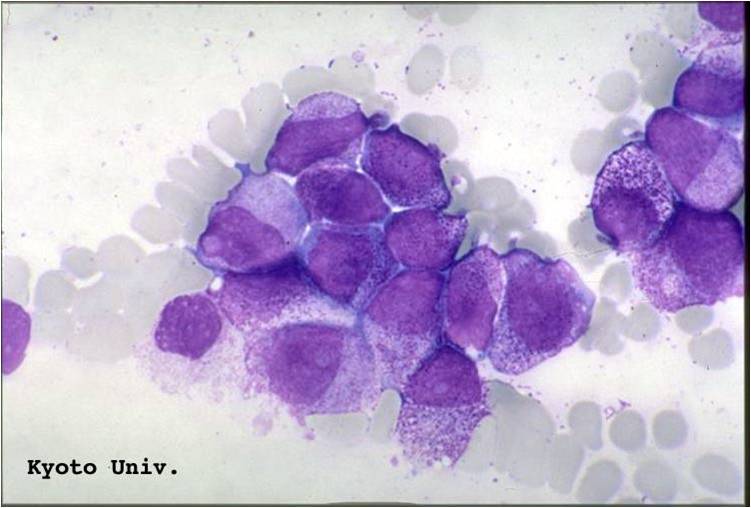
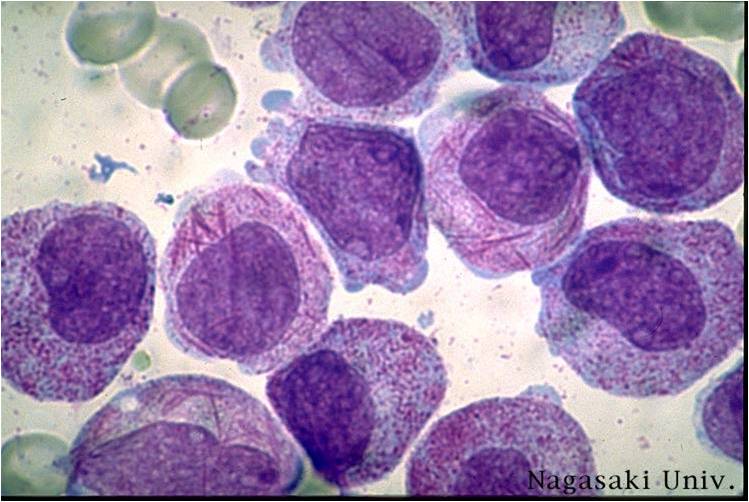
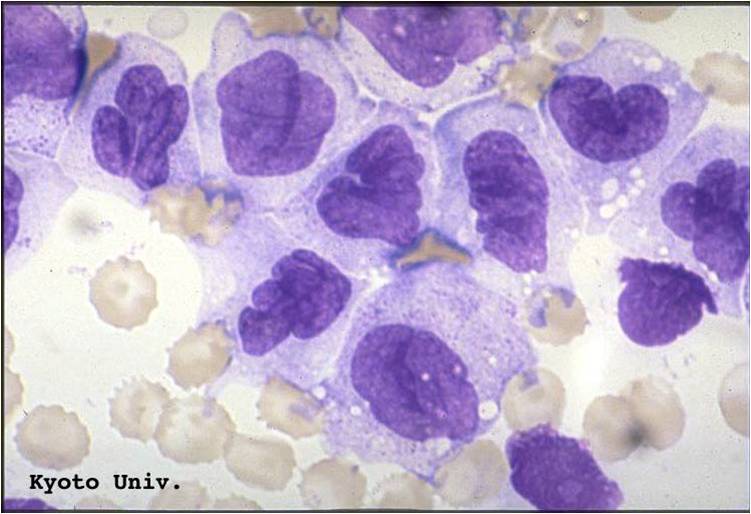
AML-M2 - presence of granules can be noted.
-
AML-M2 - large myeloblasts with prominent nucleoli.
-
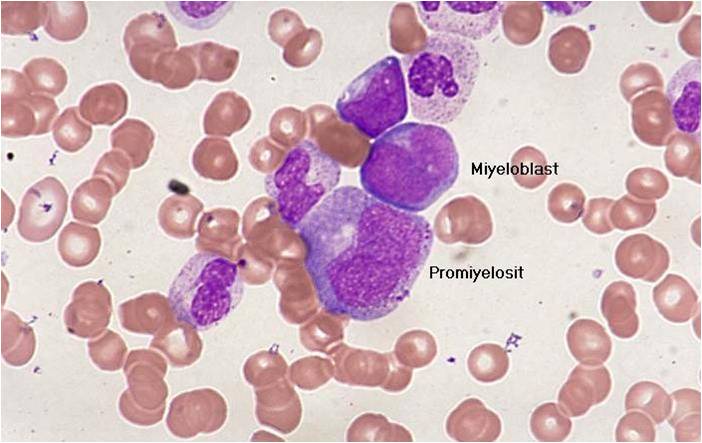
AML-M2 - maturing myeloid elements including band and segmented neutrophils in the background.
-
AML-M2 - presence of a few maturing myeloid elements.
-
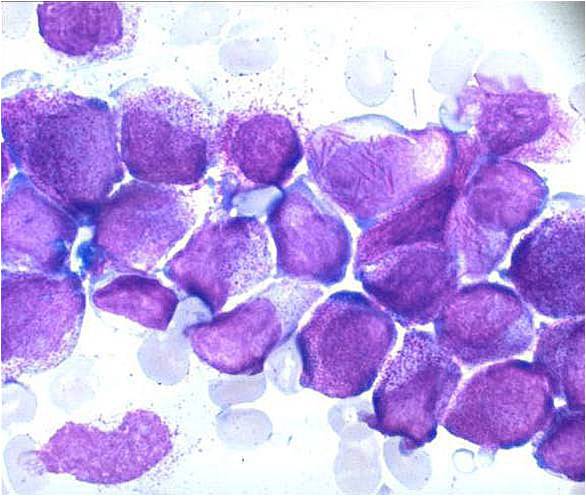
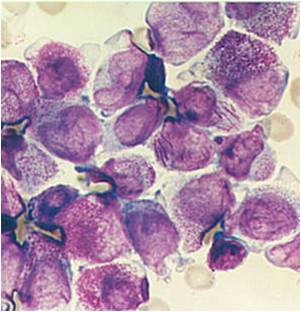
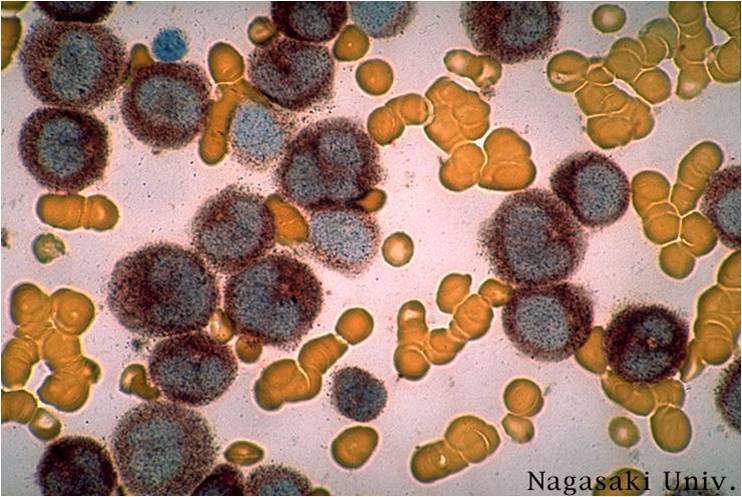
AML-M3 - also called promyelocytic leukemia. Hypergranular morphology with most cells containing abundant large granules.
-
AML-M3 - ruptured cells are releasing their granules free onto the slide. Presence of Auer rods can be noticed.
-
AML-M3 - presence of abundant fine granules.
-
AML-M3 - polarity of cytoplasmic granulation. In many cells the granules tend to polarize toward one portion of the cytoplasm and the nucleus on the opposite side.
-
AML-M3 Auer bodies.
-
AML-M3 Auer bodies.
-
AML-M3 variation.
-
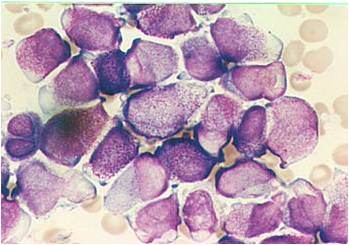
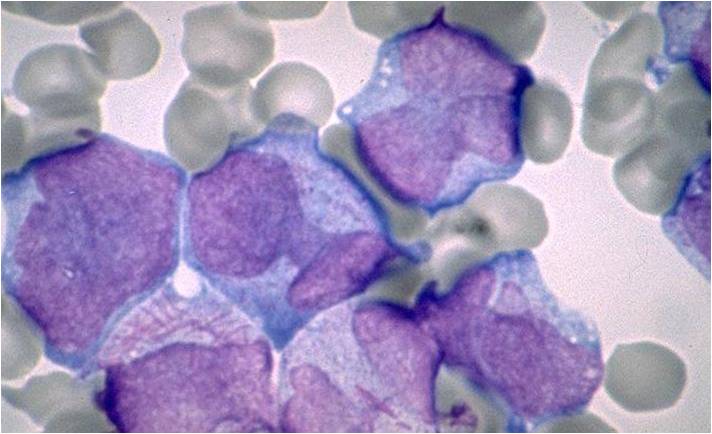
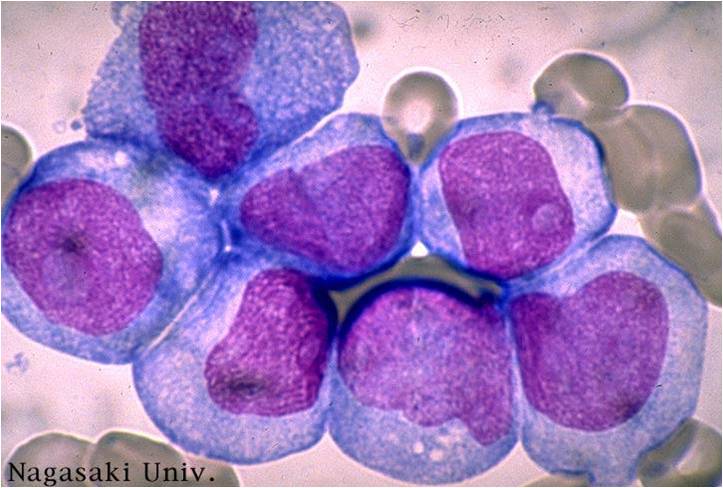
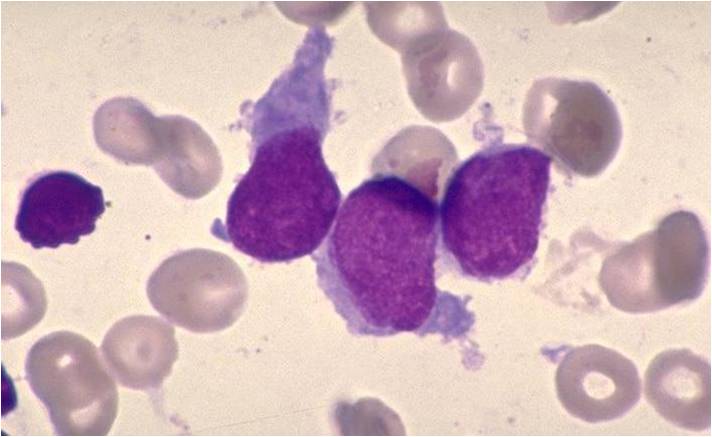
AML-M4 - large monoblasts with abundant cytoplasm and moderately to intensely basophilic. The monoblasts will have lacy nuclear chromatin and one or more predominant nucleoli.
-
AML-M5a - >80% monoblasts in the marrow.
-
AML-M5a - large monoblasts with fine nuclear chromatin and prominent nucleoli. Note the absence of Auer rods.
-
AML-M5a (alpha naphtyle acetat).
-
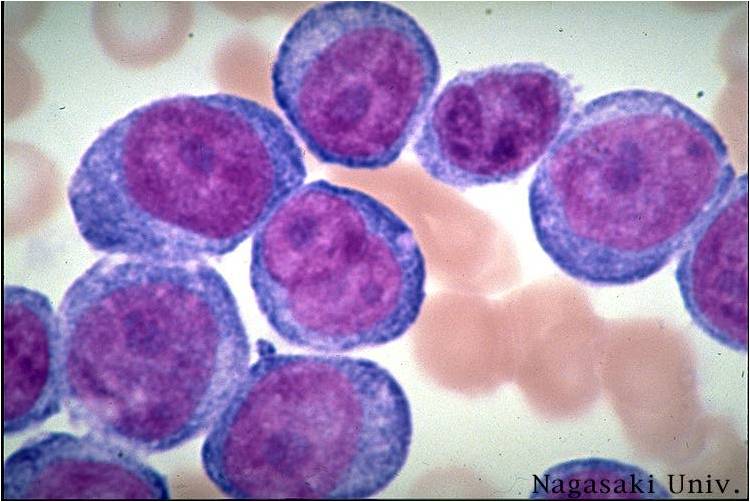
AML-M5b - <80% monoblasts in the marrow.
-
AML-M5b - moderate amounts of cytoplasm and large nuclei with fine chromatin but without prominent nucleoli. Note presence of nuclear folds and creases, which are a characteristic feature of promonocytes.
-
AML-M7 - irregular cytoplasmic border is often noted in some of the megakaryoblasts and occasionally projections resembling budding atypical platelets are present.
-
AML-M7 - megakaryoblasts are usually medium sized to large cells with a high nuclear-cytoplasmic ratio. Nuclear chromatin is dense and homogeneous.
References
- ↑ Fialkow PJ: Clonal origin of human tumors. Biochim Biophys Acta 1976;458:283–321. PMID 1067873
- ↑ Fialkow PJ, Janssen JW, Bartram CR: Clonal remissions in acute nonlymphocytic leukemia: Evidence for a multistep pathogenesis of the malignancy. Blood 1991;77:1415–1517. PMID 2009365
- ↑ Bonnet D, Dick JE: Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 1997;3:730–737. PMID 9212098
- ↑ Abeloff, Martin et al. (2004), pp. 2831–32.
- ↑ Greer, John P., et al. Wintrobe's Clinical Hematology, 11th ed. Philadelphia: Lippincott, Williams, and Wilkins, 2004. p. 2045–2062
- ↑ Melnick A, Licht JD. Deconstructing a disease: RARα its fusion partners, and their roles in the pathogenesis of acute promyelocytic leukemia. Blood 1999;93:3167–3215. PMID 10233871
- ↑ Abeloff, Martin et al. (2004), p. 2828.