Levonorgestrel (oral)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Levonorgestrel (oral) is an emergency contraceptive that is FDA approved for the prophylaxis of pregnancy following unprotected intercourse or a known or suspected contraceptive failure. To obtain optimal efficacy, the tablet should be taken as soon as possible within 72 hours of intercourse. Common adverse reactions include menstrual bleeding, nausea, lower abdominal pain, fatigue, headache, and dizziness.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Emergency Contraceptive
- Dosage: Taken orally as soon as possible within 72 hours after unprotected intercourse or a known or suspected contraceptive failure. Efficacy is better if the tablet is taken as soon as possible after unprotected intercourse. Each round tablet containing 1.5 mg of levonorgestrel.
Menorrhagia
- Dosage: Disposed as an IUD which releases 20 micrograms/day.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Levonorgestrel in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Levonorgestrel in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Levonorgestrel (oral) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Levonorgestrel in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Levonorgestrel in pediatric patients.
Contraindications
Contraindicated for use in the case of known or suspected pregnancy.
Warnings
Ectopic Pregnancy
- Ectopic pregnancies account for approximately 2% of all reported pregnancies. Up to 10% of pregnancies reported in clinical studies of routine use of progestin-only contraceptives are ectopic.
- A history of ectopic pregnancy is not a contraindication to use of this emergency contraceptive method. Healthcare providers, however, should consider the possibility of an ectopic pregnancy in women who become pregnant or complain of lower abdominal pain after taking Levonorgestrel. A follow-up physical or pelvic examination is recommended if there is any doubt concerning the general health or pregnancy status of any woman after taking Levonorgestrel.
Existing Pregnancy
- Levonorgestrel is not effective in terminating an existing pregnancy.
Effect on Menses
- Some women may experience spotting a few days after taking Levonorgestrel. Menstrual bleeding patterns are often irregular among women using progestin-only oral contraceptives and women using levonorgestrel for postcoital and emergency contraception.
- If there is a delay in the onset of expected menses beyond 1 week, consider the possibility of pregnancy.
STI/HIV
- Levonorgestrel does not protect against HIV infection (AIDS) or other sexually transmitted infections (STIs).
Physical Examination and Follow-up
- A physical examination is not required prior to prescribing Levonorgestrel. A follow-up physical or pelvic examination is recommended if there is any doubt concerning the general health or pregnancy status of any woman after taking Levonorgestrel.
Fertility Following Discontinuation
- A rapid return of fertility is likely following treatment with Levonorgestrel for emergency contraception; therefore, routine contraception should be continued or initiated as soon as possible following use of Levonorgestrel to ensure ongoing prevention of pregnancy.
Presence of FD&C Yellow
- Levonorgestrel contains FD&C Yellow #6 as a color additive.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
- Levonorgestrel tablet was studied in a randomized, double-blinded multicenter clinical trial. In this study, all women who had received at least one dose of study medication were included in the safety analysis: 1,379 women in the levonorgestrel tablet group (1 dose of 1.5 mg levonorgestrel), and 1,377 women in the levonorgestrel tablets group (2 doses of 0.75 mg levonorgestrel taken 12 hours apart). The mean age of women given levonorgestrel tablet was 27 years. The racial demographic of those enrolled was 54% Chinese, 12% Other Asian or Black, and 34% were Caucasian in each treatment group. 1.6% of women in the levonorgestrel tablet group and 1.4% in levonorgestrel tablets group were lost to follow-up.
- The most common adverse events (>10%) in the clinical trial for women receiving levonorgestrel tablet included heavier menstrual bleeding (30.9%), nausea (13.7%), lower abdominal pain (13.3%), fatigue (13.3%), and headache (10.3%). Table 1 lists those adverse events that were reported in > 4% of levonorgestrel tablet users.

Postmarketing Experience
The following adverse reactions have been identified during post-approval use of levonorgestrel tablets (2 doses of 0.75 mg levonorgestrel taken 12 hours apart). Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal Disorders
General Disorders and Administration Site Conditions
Nervous System Disorders
Reproductive System and Breast Disorders
Drug Interactions
- Drugs or herbal products that induce enzymes, including CYP3A4, that metabolize progestins may decrease the plasma concentrations of progestins, and may decrease the effectiveness of progestin-only pills. Some drugs or herbal products that may decrease the effectiveness of progestin-only pills include:
- Barbiturates
- Bosentan
- Carbamazepine
- Felbamate
- Griseofulvin
- Oxcarbazepine
- Phenytoin
- Rifampin
- St. John’s wort
- Topiramate
- Significant changes (increase or decrease) in the plasma levels of the progestin have been noted in some cases of coadministration with HIV protease inhibitors or with non-nucleoside reverse transcriptase inhibitors.
- Consult the labeling of all concurrently used drugs to obtain further information about interactions with progestin-only pills or the potential for enzyme alterations.
Use in Specific Populations
Pregnancy
- Many studies have found no harmful effects on fetal development associated with long-term use of contraceptive doses of oral progestins. The few studies of infant growth and development that have been conducted with progestin-only pills have not demonstrated significant adverse effects.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Levonorgestrel (oral) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Levonorgestrel (oral) during labor and delivery.
Nursing Mothers
- In general, no adverse effects of progestin-only pills have been found on breastfeeding performance or on the health, growth, or development of the infant. However, isolated post-marketing cases of decreased milk production have been reported. Small amounts of progestins pass into the breast milk of nursing mothers taking progestin-only pills for long-term contraception, resulting in detectable steroid levels in infant plasma.
Pediatric Use
- Safety and efficacy of progestin-only pills for long-term contraception have been established in women of reproductive age. Safety and efficacy are expected to be the same for postpubertal adolescents less than 17 years and for users 17 years and older. Use of Levonorgestrel emergency contraception before menarche is not indicated.
Geriatic Use
- This product is not intended for use in postmenopausal women.
Gender
There is no FDA guidance on the use of Levonorgestrel (oral) with respect to specific gender populations.
Race
- No formal studies have evaluated the effect of race. However, clinical trials demonstrated a higher pregnancy rate in Chinese women with both levonorgestrel tablets (2 doses of 0.75 mg levonorgestrel taken 12 hours apart) and the Yuzpe regimen (another form of emergency contraception). There was a non-statistically significant increased rate of pregnancy among Chinese women in the levonorgestrel tablet trial. The reason for this apparent increase in the pregnancy rate with emergency contraceptives in Chinese women is unknown.
Renal Impairment
- No formal studies were conducted to evaluate the effect of renal disease on the disposition of levonorgestrel tablet.
Hepatic Impairment
- No formal studies were conducted to evaluate the effect of hepatic disease on the disposition of levonorgestrel tablet.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Levonorgestrel (oral) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Levonorgestrel (oral) in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Levonorgestrel (oral) Administration in the drug label.
Monitoring
There is limited information regarding Levonorgestrel (oral) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Levonorgestrel (oral) and IV administrations.
Overdosage
- There are no data on overdosage of levonorgestrel tablet, although the common adverse event of nausea and associated vomiting may be anticipated.
Pharmacology
Mechanism of Action
- Emergency contraceptive pills are not effective if a woman is already pregnant. Levonorgestrel is believed to act as an emergency contraceptive principally by preventing ovulation or fertilization (by altering tubal transport of sperm and/or ova). In addition, it may inhibit implantation (by altering the endometrium). It is not effective once the process of implantation has begun.
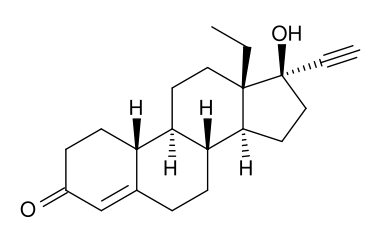
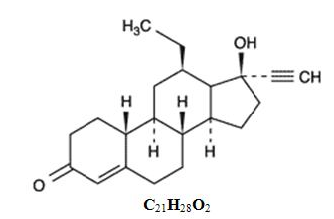
Structure
- The inactive ingredients present are colloidal silicon dioxide, corn starch, FD&C Yellow #6, lactose monohydrate, magnesium stearate, povidone. Levonorgestrel has a molecular weight of 312.45, and the following structural and molecular formulas:
Pharmacodynamics
There is limited information regarding Levonorgestrel (oral) Pharmacodynamics in the drug label.
Pharmacokinetics
Absorption
- Following a single dose administration of levonorgestrel 1.5 mg in 30 women under fasting conditions, maximum plasma concentrations of levonorgestrel of 19.1 ng/mL were reached at 1.7 hours.

- Cmax = maximum concentration
- AUCt = area under the drug concentration curve from time 0 to time of last determinable concentration
- AUCinf = area under the drug concentration curve from time 0 to infinity
- Tmax = time to maximum concentration
- t1/2 = elimination half life
- N = 29
- median (range)
- Effect of Food: The effect of food on the rate and the extent of levonorgestrel absorption following single oral administration of levonorgestrel 1.5 mg has not been evaluated.
Distribution
- The apparent volume of distribution of levonorgestrel is reported to be approximately 1.8 L/kg. It is about 97.5 to 99% protein-bound, principally to sex hormone binding globulin (SHBG) and, to a lesser extent, serum albumin.
Metabolism
- Following absorption, levonorgestrel is conjugated at the 17β-OH position to form sulfate conjugates and, to a lesser extent, glucuronide conjugates in plasma. Significant amounts of conjugated and unconjugated 3α, 5β-tetrahydrolevonorgestrel are also present in plasma, along with much smaller amounts of 3α, 5α-tetrahydrolevonorgestrel and 16βhydroxylevonorgestrel. Levonorgestrel and its phase I metabolites are excreted primarily as glucuronide conjugates. Metabolic clearance rates may differ among individuals by several-fold, and this may account in part for the wide variation observed in levonorgestrel concentrations among users.
Excretion
- About 45% of levonorgestrel and its metabolites are excreted in the urine and about 32% are excreted in feces, mostly as glucuronide conjugates.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity
- There is no evidence of increased risk of cancer with short-term use of progestins. There was no increase in tumorgenicity following administration of levonorgestrel to rats for 2 years at approximately 5 mcg/day, to dogs for 7 years at up to 0.125 mg/kg/day, or to rhesus monkeys for 10 years at up to 250 mcg/kg/day. In another 7 year dog study, administration of levonorgestrel at 0.5 mg/kg/day did increase the number of mammary adenomas in treated dogs compared to controls. There were no malignancies.
Genotoxicity
- Levonorgestrel was not found to be mutagenic or genotoxic in the Ames Assay, in vitro mammalian culture assays utilizing mouse lymphoma cells and Chinese hamster ovary cells, and in an in vivo micronucleus assay in mice.
Fertility
- There are no irreversible effects on fertility following cessation of exposures to levonorgestrel or progestins in general.
Clinical Studies
- A double-blind, randomized, multicenter, multinational study evaluated and compared the efficacy and safety of three different regimens for emergency contraception. Subjects were enrolled at 15 sites in 10 countries; the racial/ethnic characteristics of the study population overall were 54% Chinese, 34% Caucasian, and 12% Black or Asian (other than Chinese). 2,381 healthy women with a mean age of 27 years, who needed emergency contraception within 72 hours of unprotected intercourse were involved and randomly allocated into one of the two levonorgestrel groups. A single dose of 1.5 mg of levonorgestrel (levonorgestrel tablet) was administered to women allocated into group 1. Two doses of 0.75 mg levonorgestrel 12 hours apart (levonorgestrel tablets) were administered to women in group 2. In the levonorgestrel tablet group, 16 pregnancies occurred in 1,198 women and in the levonorgestrel tablets group, 20 pregnancies occurred in 1,183 women. The number of pregnancies expected in each group was calculated based on the timing of intercourse with regard to each woman’s menstrual cycle. Among women receiving levonorgestrel tablet, 84% of expected pregnancies were prevented and among those women taking levonorgestrel tablets, 79% of expected pregnancies were prevented. The expected pregnancy rate of 8% (with no contraceptive use) was reduced to approximately 1% with levonorgestrel tablet. Emergency contraceptives are not as effective as routine contraception since their failure rate, while low based on a single use, would accumulate over time with repeated use.
- In the clinical study, bleeding disturbances were the most common adverse event reported after taking the levonorgestrel-containing regimens. More than half of the women had menses within two days of the expected time; however, 31% of women experienced change in their bleeding pattern during the study period; 4.5% of women had menses more than 7 days after the expected time.
How Supplied
- Levonorgestrel (Levonorgestrel) tablet 1.5 mg is available in a PVC/aluminum foil blister package. The tablet is supplied as a peach, round tablet and is embossed with 287 on one side and WATSON on the other side.
Storage
- Store Levonorgestrel at 20° to 25°C (68° to 77°F)
Images
Drug Images
{{#ask: Page Name::Levonorgestrel (oral) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Levonorgestrel (oral) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Take Levonorgestrel as soon as possible and not more than 72 hours after unprotected intercourse or a known or suspected contraceptive failure.
- If you vomit within two hours of taking the tablet, immediately contact your healthcare provider to discuss whether to take another tablet.
- Seek medical attention if you experience severe lower abdominal pain 3 to 5 weeks after taking Levonorgestrel, in order to be evaluated for an ectopic pregnancy.
- After taking Levonorgestrel, consider the possibility of pregnancy if your period is delayed more than one week beyond the date you expected your period.
- Do not use Levonorgestrel as routine contraception.
- Levonorgestrel is not effective in terminating an existing pregnancy.
- Levonorgestrel does not protect against HIV-infection (AIDS) and other sexually transmitted diseases/infections.
- For women younger than age 17 years, Levonorgestrel is available only by prescription.
- Levonorgestrel tablets contain FD&C Yellow #6 as a color additive.
Precautions with Alcohol
Alcohol-Levonorgestrel interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Levonorgestrel (oral) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Levonorgestrel (oral) |Label Name=NextChoice Package.png
}}
{{#subobject:
|Label Page=Levonorgestrel (oral) |Label Name=NextChoice Package (Posterior).png
}}