Miglustat
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Miglustat is a glucosylceramide synthase inhibitor that is FDA approved for the {{{indicationType}}} of adult patients with mild/moderate type 1 Gaucher disease for whom enzyme replacement therapy is not a therapeutic option. Common adverse reactions include diarrhea, weight loss, stomach pain, gas, nausea, vomiting, headache, migraine, tremor, leg cramps, dizziness, weakness, vision problems, thrombocytopenia, muscle cramps, back pain, constipation, dry mouth, heaviness in arms and legs, memory loss, unsteady walking, anorexia, indigestion, paresthesia, stomach bloating, stomach pain not related to food, and menstrual changes.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Type 1 Gaucher Disease
- Zavesca® is a glucosylceramide synthase inhibitor indicated as monotherapy for the treatment of adult patients with mild to moderate type 1 Gaucher disease for whom enzyme replacement therapy is not a therapeutic option (e.g. due to allergy, hypersensitivity, or poor venous access).
- The recommended dose for the treatment of adult patients with type 1 Gaucher disease is one 100 mg capsule administered orally three times a day at regular intervals. If a dose is missed, the next Zavesca capsule should be taken at the next scheduled time.
- It may be necessary to reduce the dose to one 100 mg capsule once or twice a day in some patients due to adverse reactions, such as tremor or diarrhea.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Miglustat in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Miglustat in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Miglustat in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Miglustat in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Miglustat in pediatric patients.
Contraindications
- None.
Warnings
Precautions
- Therapy should be directed by physicians knowledgeable in the management of patients with Gaucher disease.
- Peripheral Neuropathy
- In clinical trials, cases of peripheral neuropathy have been reported in 3% of Gaucher's patients treated with Zavesca. All patients receiving Zavesca treatment should undergo baseline and repeat neurological evaluations at approximately 6-month intervals. Patients who develop symptoms of peripheral neuropathy such as pain, weakness, numbness and tingling should have a careful re-assessment of the risk/benefit of Zavesca therapy, and cessation of treatment may be considered.
- Tremor
- Approximately 30% of patients have reported tremor or exacerbation of existing tremor on treatment. These tremors were described as an exaggerated physiological tremor of the hands. Tremor usually began within the first month of therapy and in many cases resolved between 1 to 3 months during treatment. Reduce dose to ameliorate tremor or discontinue treatment if tremor does not resolve within days of dose reduction.
- Diarrhea and Weight Loss
- Diarrhea and weight loss were common in clinical studies of patients treated with Zavesca, occurring in approximately 85% and up to 65% of treated patients, respectively. Diarrhea appears to be the result of the inhibitory activity of Zavesca on intestinal disaccharidases such as sucrase-isomaltase in the gastrointestinal tract leading to reduced absorption of dietary disaccharides in the small intestine, with a resultant osmotic diarrhea. It is unclear if weight loss results from the diarrhea and associated gastrointestinal complaints, a decrease in food intake, or a combination of these or other factors. The incidence of weight loss was most evident in the first 12 months of treatment. Diarrhea decreased over time with continued Zavesca treatment, and may respond to individualized diet modification (e.g., reduction of sucrose, lactose and other carbohydrate intake), to taking Zavesca between meals, and/or to anti-diarrheal medications, most commonly loperamide. Patients may be instructed to avoid high carbohydrate content foods during treatment with Zavesca if they present with diarrhea.
- Patients with persistent gastrointestinal events that continue during treatment with Zavesca, and who do not respond to usual interventions (e.g. diet modification), should be evaluated to determine whether significant underlying gastrointestinal disease is present. The safety of treatment with Zavesca has not been evaluated in patients with significant gastrointestinal disease, such as inflammatory bowel disease, and continued treatment of these patients with Zavesca should occur only after consideration of the risks and benefits of continued treatment.
- Reductions in Platelet Count
- In clinical trials evaluating the use of Zavesca for treatment of indications other than type 1 Gaucher disease, mild reductions in platelet counts without association with bleeding were observed in some patients; approximately 40% of patients in this trial had low platelet counts (defined as below 150 × 109/L) before starting treatment with Zavesca. Monitoring of platelet counts is recommended in patients with type 1 Gaucher disease. Mild reductions in platelet counts without association with bleeding were observed in patients with type 1 Gaucher disease who were switched from enzyme replacement therapy (ERT) to Zavesca.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The data described below reflect exposure of 80 patients with type 1 Gaucher disease in two open-label, uncontrolled, monotherapy trials, one open-label, active-controlled trial, and two extensions, who received Zavesca at doses ranging from 50mg to 200 mg three times daily. Patients were aged 18 to 69 years at first treatment. The population was evenly distributed by gender.
- The most common serious adverse reaction reported with Zavesca treatment in clinical trials was peripheral neuropathy.
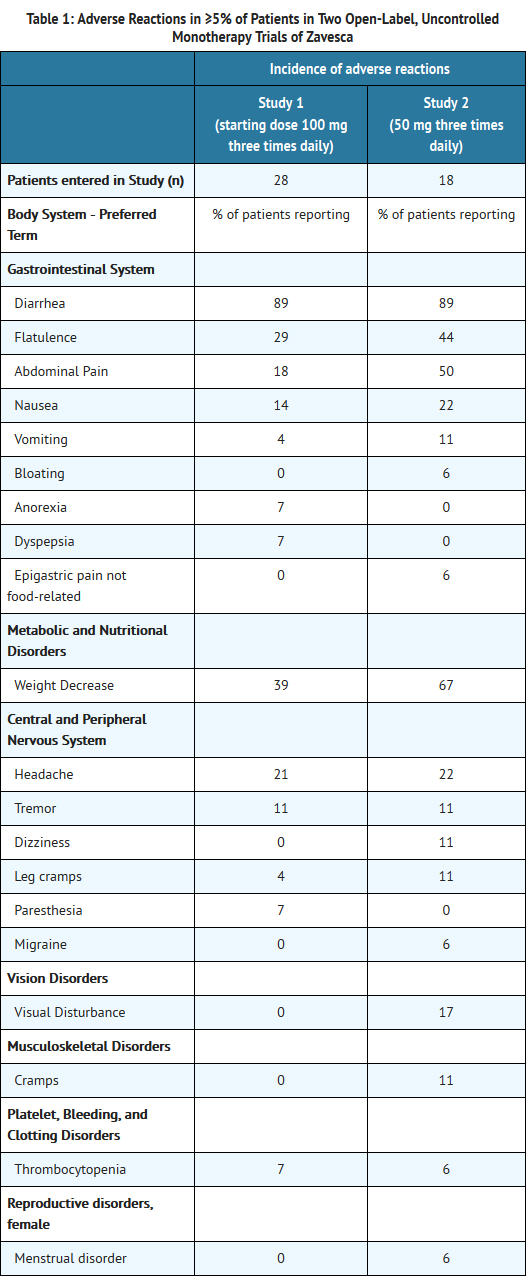
- The most commonly reported adverse reactions in patients treated with Zavesca (occuring in ≥5%) that were considered related to Zavesca are shown in Tables 1 and 2.
- In two open-label, uncontrolled monotherapy trials, adult type 1 Gaucher disease patients were treated with Zavesca at a starting dose of 100 mg three times daily (dose range 100 to 200 mg three times daily) for up to 12 months in 28 patients [Study 1], or at a dose of 50 mg three times daily for up to 6 months in 18 patients [Study 2]. Table 1 below lists adverse reactions that occurred during the trials in ≥5% of patients.
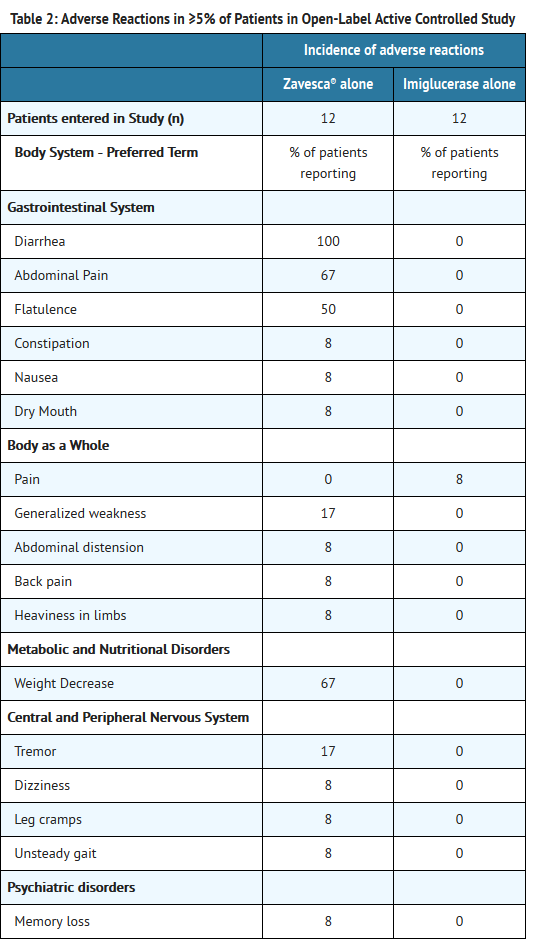
- In an open-label, active-controlled study, 36 adult type 1 Gaucher disease patients were treated with Zavesca, imiglucerase, or Zavesca plus imiglucerase [Study 3] for up to 12 months. Table 2 lists adverse reactions that occurred during the trial in ≥5% of patients.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Miglustat in the drug label.
Drug Interactions
- While co-administration of Zavesca appeared to increase the clearance of imiglucerase by 70%, these results are not conclusive because of the small number of patients studied and because patients took variable doses of imiglucerase.
Use in Specific Populations
Pregnancy
- Pregnancy Category C
- Risk Summary
- There are no adequate and well controlled studies with Zavesca in pregnant women. However, animal reproduction studies have been conducted for Zavesca. In these animal studies, decreased live births and decreased fetal weight were observed in rats orally dosed with miglustat prior to mating and during organogenesis at doses with exposures at and greater than 2 times the human therapeutic systemic exposure. Maternal death and decreased body weight gain were observed in rabbits orally dosed with miglustat during organogenesis at doses with exposures less than the human therapeutic systemic exposure. Zavesca should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Clinical Considerations
- Disease-associated maternal and embryo-fetal risk
- Women with Type 1 Gaucher disease have an increased risk of spontaneous abortion, especially if disease symptoms are not treated and controlled pre-conception and during a pregnancy. Pregnancy may exacerbate existing Type 1 Gaucher disease symptoms or result in new disease manifestations. Type 1 Gaucher disease manifestations may lead to adverse pregnancy outcomes including, hepatosplenomegaly which can interfere with the normal growth of a pregnancy and thrombocytopenia which can lead to increased bleeding and possible hemorrhage.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Miglustat in women who are pregnant.
Labor and Delivery
- Dystocia and delayed parturition were observed in rats dosed with miglustat gestation day 6 through lactation at systemic exposure ≥2 times the human therapeutic systemic exposure.
Nursing Mothers
- It is not known whether miglustat is present in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from miglustat, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the lactating woman.
Pediatric Use
- The safety and effectiveness of Zavesca in pediatric patients have not been established.
- In a combined clinical trial safety data set of 45 patients less than 18 years of age exposed to Zavesca in indications other than type 1 Gaucher disease, the median weight and height percentiles adjusted for age and gender decreased during the first year of treatment but then stabilized. The mean length of exposure in these studies ranged from 2 to 2.6 years; some pediatric patients were exposed for up to 4 years. However, the effect of Zavesca on long-term gain in weight and height in pediatric patients is unclear.
Geriatic Use
- Clinical studies of Zavesca did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently than younger patients. Other reported clinical experience has not identified differences in responses between elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, and cardiac function and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Miglustat with respect to specific gender populations.
Race
There is no FDA guidance on the use of Miglustat with respect to specific racial populations.
Renal Impairment
- Miglustat is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function.
- In patients with mild renal impairment (adjusted creatinine clearance 50-70 mL/min/1.73 m2), Zavesca administration should commence at a dose of 100 mg twice per day.
- In patients with moderate renal impairment (adjusted creatinine clearance of 30-50 mL/min/1.73 m2), Zavesca administration should commence at a dose of 100 mg once a day.
- Use of Zavesca in patients with severe renal impairment (creatinine clearance < 30 mL/min/1.73 m2) is not recommended.
- Since elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function. The impact of hemodialysis on the disposition of Zavesca has not been investigated.
Hepatic Impairment
There is no FDA guidance on the use of Miglustat in patients with hepatic impairment.
Females of Reproductive Potential and Males
- Infertility
- No effect on sperm concentration, motility, or morphology was seen in 7 healthy adult men who received miglustat 100 mg, orally, twice daily for 6 weeks. Decreased spermatogenesis with altered sperm morphology and motility and decreased fertility were observed in rats orally dosed with miglustat 14 days prior to mating with doses at exposures less than the human therapeutic systemic exposure based on body surface area comparisons (mg/m2). Decreased spermatogenesis was reversible in rats following 6 weeks of drug withdrawal.
Immunocompromised Patients
There is no FDA guidance one the use of Miglustat in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Miglustat in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Miglustat in the drug label.
Overdosage
Chronic Overdose
There is limited information regarding Chronic Overdose of Miglustat in the drug label.
Pharmacology
| |
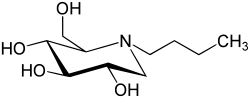
Miglustat
| |
| Systematic (IUPAC) name | |
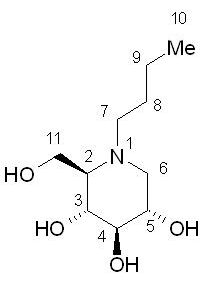
| (2R,3R,4R,5S)-1-butyl-2-(hydroxymethyl)piperidine-3,4,5-triol | |
| Identifiers | |
| CAS number | |
| ATC code | A16 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 219.28 g/mol |
| SMILES | & |
| Synonyms | 1,5-(butylimino)-1,5-dideoxy-D-glucitol, N-butyl-deoxynojirimycin |
| Pharmacokinetic data | |
| Bioavailability | 97% |
| Protein binding | Nil |
| Metabolism | Nil |
| Half life | 6–7 hours |
| Excretion | Renal, unchanged |
| Therapeutic considerations | |
| Licence data |
|
| Pregnancy cat. |
X(US) |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | Oral |
Mechanism of Action
- Type 1 Gaucher disease is caused by a functional deficiency of glucocerebrosidase, the enzyme that mediates the degradation of the glycosphingolipid glucosylceramide.
- Miglustat functions as a competitive and reversible inhibitor of the enzyme glucosylceramide synthase, the initial enzyme in a series of reactions which results in the synthesis of most glycosphingolipids.
- Zavesca helps reduce the rate of glycosphingolipid biosynthesis so that the amount of glycosphingolipid substrate is reduced to a level which allows the residual activity of the deficient glucocerebrosidase enzyme to be more effective (substrate reduction therapy). In vitro and in vivo studies have shown that miglustat can reduce the synthesis of glucosylceramide-based glycosphingolipids.
Structure
- Zavesca (miglustat capsules, 100 mg) is an inhibitor of the enzyme glucosylceramide synthase, which is a glucosyl transferase enzyme responsible for the first step in the synthesis of most glycosphingolipids. Zavesca is an N-alkylated imino sugar, a synthetic analog of D-glucose.
- The chemical name for miglustat is 1,5-(butylimino)-1,5-dideoxy-D-glucitol with the chemical formula C10H21NO4 and a molecular weight of 219.28.
- Miglustat is a white to off-white crystalline solid and has a bitter taste. It is highly soluble in water (>1000 mg/mL as a free base).
- Zavesca is supplied in hard gelatin capsules each containing 100 mg miglustat for oral administration. Each Zavesca 100 mg capsule also contains sodium starch glycollate, povidone (K30), and magnesium stearate. Ingredients in the capsule shell include gelatin and titanium dioxide, and the shells are printed with edible ink consisting of black iron oxide and shellac.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Miglustat in the drug label.
Pharmacokinetics
- Absorption: After a 100 mg oral dose, the time to maximum observed plasma concentration of miglustat (tmax) ranged from 2 to 2.5 hours in Gaucher patients. Plasma concentrations show a biexponential decline, characterized by a short distribution phase and a longer elimination phase. The effective half-life of miglustat is approximately 6 to 7 hours, which predicts that steady-state will be achieved by 1.5 to 2 days following the start of three times daily dosing.
- Miglustat, dosed at 50 and 100 mg three times daily in Gaucher patients, exhibits dose-proportional pharmacokinetics. The pharmacokinetics of miglustat were not altered after repeated dosing three times daily for up to 12 months.
- In healthy subjects, co-administration of Zavesca with food results in a decrease in the rate of absorption of miglustat (maximum plasma concentration [Cmax] was decreased by 36% and tmax delayed 2 h) but had no statistically significant effect on the extent of absorption of miglustat (area-under-the-plasma-concentration time curve [AUC] was decreased by 14%). The mean oral bioavailability of a 100-mg miglustat capsule is about 97% relative to an oral solution administered under fasting conditions. The pharmacokinetics of miglustat were similar between adult type 1 Gaucher disease patients and healthy subjects after a single dose administration of miglustat 100 mg.
- Distribution: Miglustat does not bind to plasma proteins. Mean apparent volume of distribution of miglustat is 83-105 liters in Gaucher patients. At steady state, the concentration of miglustat in cerebrospinal fluid of six non-Gaucher patients was 31.4-67.2% of that in plasma, indicating that miglustat crosses the blood brain barrier.
- Metabolism and Excretion: The major route of excretion of miglustat is via kidney. Following administration of a single dose of 100 mg 14C-miglustat to healthy volunteers, 83% of the radioactivity was recovered in urine and 12% in feces. In healthy subjects, 67% of the administered dose was excreted unchanged in urine over 72 hours. The most abundant metabolite in urine was miglustat glucuronide accounting for 5% of the dose. The terminal half-life of radioactivity in plasma was 150 hours, suggesting the presence of one or more metabolites with a prolonged half-life. The metabolite accounting for this observation has not been identified, but may accumulate and reach concentrations exceeding those of miglustat at steady state.
- Specific Populations
- Gender: There was no statistically significant gender difference in miglustat pharmacokinetics, based on pooled data analysis.
- Race: Ethnic differences in miglustat pharmacokinetics have not been evaluated in Gaucher patients. However, apparent oral clearance of miglustat in patients of Ashkenazi Jewish descent was not statistically different to that in others (1 Asian and 15 Caucasians), based on a cross-study analysis.
- Hepatic Impairment: No studies have been performed to assess the pharmacokinetics of miglustat in patients with hepatic impairment.
- Renal Impairment: Limited data in non-Gaucher patients with impaired renal function indicate that the apparent oral clearance (CL/F) of miglustat decreases with decreasing renal function. While the number of subjects with mild and moderate renal impairment was very small, the data suggest an approximate decrease in the apparent oral clearance of 40% and 60% respectively, in mild and moderate renal impairment, justifying the need to decrease the dosing of miglustat in such patients dependent upon creatinine clearance levels.
- Data in severe renal impairment are limited to two patients with creatinine clearances in the range 18-29 mL/min and cannot be extrapolated below this range. These data suggest a decrease in CL/F by at least 70% in patients with severe renal impairment.
- Drug Interaction Studies
- Miglustat does not inhibit the metabolism of various substrates of cytochrome P450 enzymes including, CYP1A2, CYP2A6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP3A4 and CYP4A11 in vitro; consequently significant interactions via inhibition of these enzymes are unlikely with drugs that are substrates of cytochrome P450 enzymes.
- Drug interaction between Zavesca (miglustat 100 mg orally three times daily) and imiglucerase 7.5 or 15 U/kg/day was assessed in imiglucerase-stabilized patients after one month of co-administration. There was no significant effect of imiglucerase on the pharmacokinetics of miglustat, with the co-administration of imiglucerase and miglustat resulting in a 22% reduction in Cmax and a 14% reduction in the AUC for miglustat. While Zavesca appeared to increase the clearance of imiglucerase by 70%, these results are not conclusive because of the small number of subjects studied and because patients took variable doses of imiglucerase.
- Concomitant therapy with loperamide during clinical trials did not appear to significantly alter the pharmacokinetics of miglustat.
Nonclinical Toxicology
- Carcinogenesis: Two-year carcinogenicity studies have been conducted with miglustat in CD-1 mice at oral doses up to 500 mg/kg/day and in Sprague Dawley rats at oral doses up to 180 mg/kg/day. Oral administration of miglustat for 104 weeks produced mucinous adenocarcinomas of the large intestine at 210, 420 and 500 mg/kg/day (about 3, 6 and 7 times the recommended human dose, respectively, based on the body surface area) in male mice and at 420 and 500 mg/kg/day (about 6 and 7 times the recommended human dose, based on the body surface area) in female mice. The adenocarcinomas were considered rare in CD-1 mice and occurred in the presence of inflammatory and hyperplastic lesions in the large intestine of both males and females. In rats, oral administration of miglustat for 100 weeks produced increased incidences of interstitial cell adenomas of the testis at 30, 60 and 180 mg/kg/day (about 1, 2 and 5 times the recommended human dose, respectively, based on the body surface area).
- Mutagenesis: Miglustat was not mutagenic or clastogenic in a battery of in vitro and in vivo assays including the bacterial reverse mutation (Ames), chromosomal aberration (in human lymphocytes), gene mutation in mammalian cells (Chinese hamster ovary), and mouse micronucleus assays.
- Impairment of Fertility: Male rats, given 20 mg/kg/day miglustat by (systemic exposure less than the human therapeutic systemic exposure based on body surface area comparisons, mg/m2) oral gavage 14 days prior to mating, had decreased spermatogenesis with altered sperm morphology and motility and decreased fertility. Decreased spermatogenesis was reversible following 6 weeks of drug withdrawal. A higher dose of 60 mg/kg/day (2 times the human therapeutic systemic exposure, based on body surface area comparison, mg/m2) resulted in seminiferous tubule and testicular atrophy/degeneration.
- Female rats were given oral gavage doses of 20, 60, 180 mg/kg/day beginning 14 days before mating and continuing through gestation. Effects observed at 20 mg/kg/day (systemic exposure less than the human therapeutic systemic exposure, based on body surface area comparisons) included decreased corpora lutea, increased postimplantation loss, and decreased live births.
- Animal Toxicology and/or Pharmacology
- Histopathology findings in the absence of clinical signs in the central nervous system of the monkey (brain, spine) that included vascular mineralization, in addition to mineralization and necrosis of white matter were observed at >750 mg/kg/day (4 times the human therapeutic systemic exposure based on area-under-the-plasma-concentration curve [AUC] comparisons) in a 52-week oral toxicity study using doses of 750 and 2000 mg/kg/d. Vacuolization of white matter was observed in rats dosed orally by gavage at ≥ 180 mg/kg/d (6 times the human therapeutic exposure based on surface area comparisons, mg/m2) in a 4-week study using doses of 180, 840, and 4200 mg/kg/d. Vacuolization can sometimes occur as an artifact of tissue processing. Findings in dogs included tremor and absent corneal reflexes at 105 mg/kg/day (10 times the human therapeutic systemic exposure, based on body surface area comparisons, mg/m2) after a 4-week oral gavage toxicity study using doses of 35, 70, 105, and 140 mg/kg/d. Ataxia, diminished/absent pupillary, palpebral, or patellar reflexes were observed in a dog at ≥495 mg/kg/day (50 times the human therapeutic systemic exposure based on body surface area comparisons, mg/m2), in a 2-week oral gavage toxicity study using doses of 85, 165, 495, and 825 mg/kg/d.
- Cataracts were observed in rats at ≥180 mg/kg/day (4 times the human therapeutic systemic exposure, based on AUC) in a 52-week oral gavage toxicity study using doses of 180, 420, 840, and 1680 mg/kg/d.
- Gastrointestinal necrosis, inflammation, and hemorrhage were observed in dogs at ≥ 85 mg/kg/day (9 times the human therapeutic systemic exposure based on body surface area comparisons, mg/m2) after a 2-week oral (capsule) toxicity study using doses of 85, 165, 495, and 825 mg/kg/d. Similar GI toxicity occurred in rats at 1200 mg/kg/day (7 times the human therapeutic systemic exposure, based on AUC) in a 26-week oral gavage toxicity study using doses of 300, 600, and 1200 mg/kg/d. In monkeys, similar GI toxicity occurred at ≥750 mg/kg/day (6 times the human therapeutic systemic exposure based on AUC) following a 52-week oral gavage toxicity study using doses of 750 and 2000 mg/kg/d.
Clinical Studies
- The efficacy of Zavesca in type 1 Gaucher disease has been investigated in two open-label, uncontrolled trials and one randomized, open-label, active-controlled trial with enzyme replacement given as imiglucerase. Patients who received Zavesca were treated with doses ranging from 100 to 600 mg a day, although the majority of patients were maintained on doses between 200 to 300 mg a day. Efficacy parameters included the evaluation of liver and spleen organ volume, hemoglobin concentration, and platelet count. A total of 80 patients were exposed to Zavesca during the three trials and their extension period.
- Open-Label Uncontrolled Monotherapy Trials
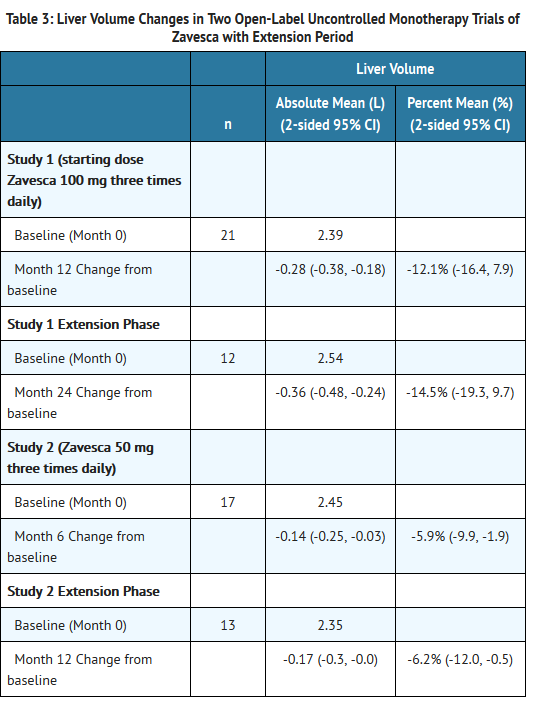
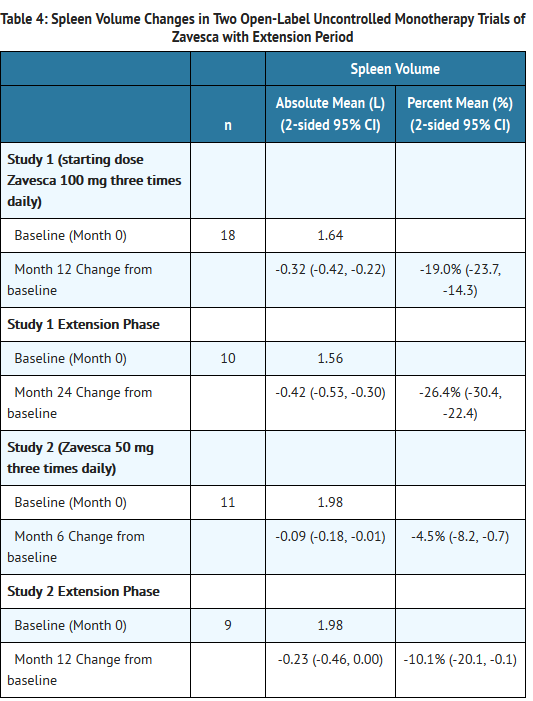
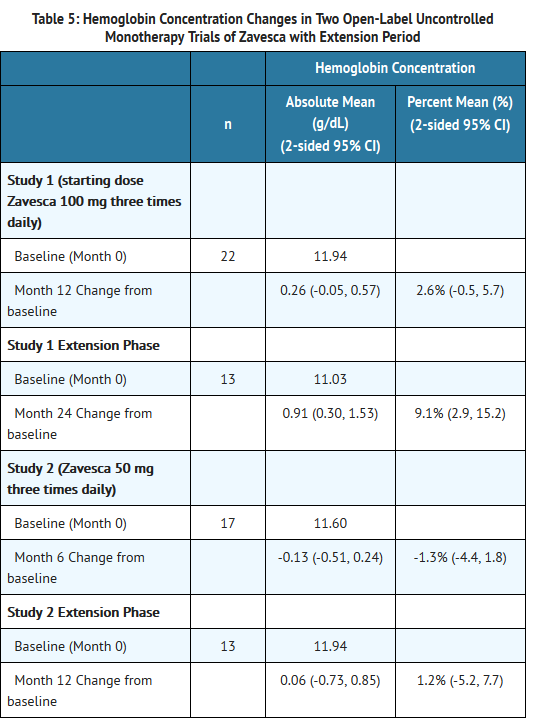
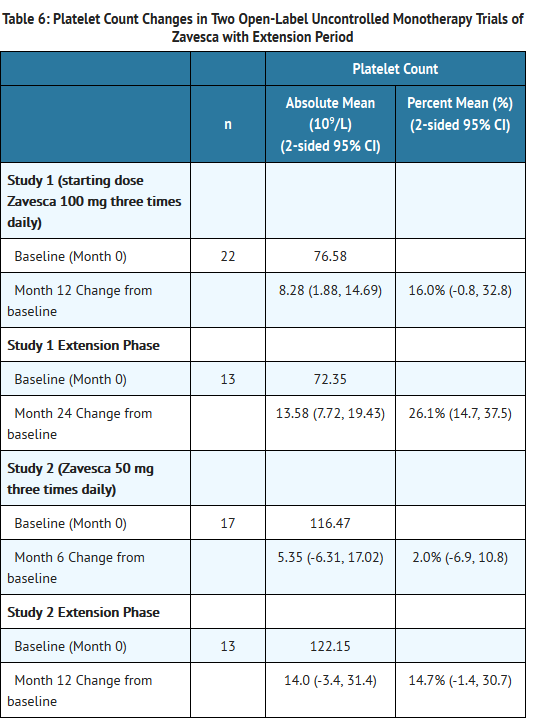
- In Study 1, Zavesca was administered at a starting dose of 100 mg three times daily for 12 months (dose range of 100 once-daily to 200 mg three times daily) to 28 adult patients with type 1 Gaucher disease, who were unable to receive enzyme replacement therapy and who had not taken enzyme replacement therapy in the preceding 6 months. Twenty-two patients completed the trial. After 12 months of treatment, the results showed significant mean percent reductions from baseline in liver volume of 12% and spleen volume of 19%, a non-significant increase from baseline in mean absolute hemoglobin concentration of 0.26 g/dL and a mean absolute increase from baseline in platelet counts of 8 × 109/L (See Tables 3-6).
- In Study 2, Zavesca was administered at a dose of 50 mg three times daily for 6 months to 18 adult patients with type 1 Gaucher disease who were unable to receive enzyme replacement therapy and who had not taken enzyme replacement therapy in the preceding 6 months. Seventeen patients completed the trial. After 6 months of treatment, the results showed significant mean percent reductions from baseline in liver volume of 6% and spleen volume of 5%. There was a non-significant mean absolute decrease from baseline in hemoglobin concentration of 0.13 g/dL and a non-significant mean absolute increase from baseline in platelet counts of 5 × 109/L (See Tables 3-6).
- Extension Period
- Eighteen patients were enrolled in a 12-month extension to Study 1. A subset of patients continuing in the extension had larger mean baseline liver volumes, and lower mean baseline platelet counts and hemoglobin concentrations than the original study population (See Tables 3-6). After a total of 24 months of treatment, there were significant mean decreases from baseline in liver and spleen organ volumes of 15% and 27%, respectively, and significant mean absolute increases from baseline in hemoglobin concentration and platelet count of 0.9 g/dL and 14 × 109/L, respectively (See Tables 3-6).
- Sixteen patients were enrolled in a 6-month extension to Study 2. After a total of 12 months of treatment, there was a mean decrease from baseline in spleen organ volume of 10%, whereas the mean percent decrease in liver organ volume remained at 6%. There were no significant changes in hemoglobin concentrations or platelet counts (See Tables 3-6).
- Liver volume results from Studies 1 and 2 and their extensions are summarized in Table 3:
- Open-Label Active-Controlled Trial
- Study 3 was an open-label, randomized, active-controlled study of 36 adult patients with type 1 Gaucher disease, who had been receiving enzyme replacement therapy with imiglucerase for a minimum of 2 years prior to study entry. Patients were randomized 1:1:1 to one of three treatment groups, as follows:
- Zavesca 100 mg three times daily alone
- imiglucerase (patient's usual dose) alone
- Zavesca 100 mg three times daily and imiglucerase (usual dose)
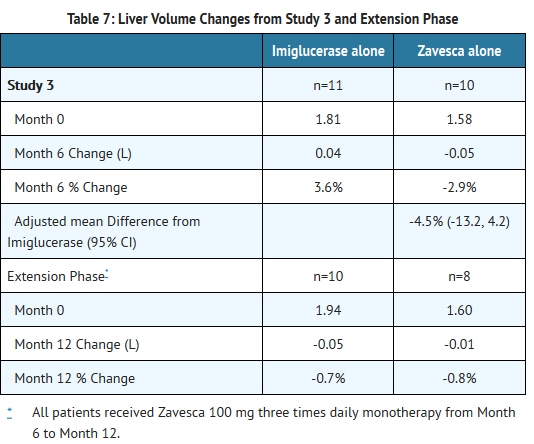
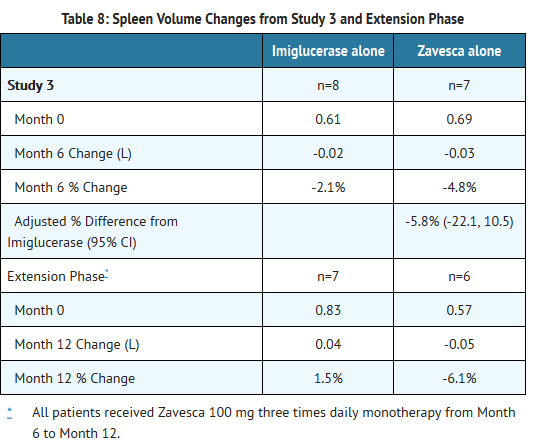
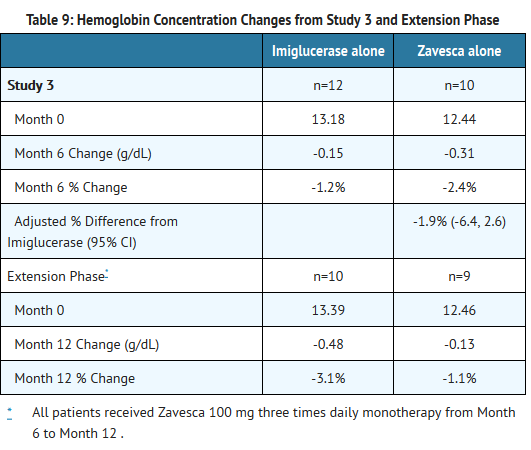
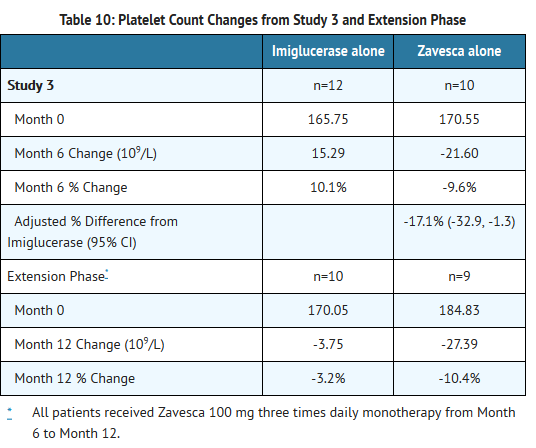
- Patients were treated for 6 months, and 33 patients completed the study. Because Zavesca is only indicated as monotherapy, the results for the monotherapy arms are described below. At Month 6, the results showed a decrease in mean percent change in liver volume in the Zavesca treatment group compared to the imiglucerase alone group. There were no significant differences between the groups for mean absolute changes in liver and spleen volume and hemoglobin concentration. However, there was a significant difference between the Zavesca alone and imiglucerase alone groups in platelet counts at Month 6, with the Zavesca alone group having a mean absolute decrease in platelet count of 21.6 × 109/L and the imiglucerase alone group having a mean absolute increase in platelet count of 10.1 × 109/L (See Tables 7-10).
- Extension period
- Twenty-nine patients were enrolled in a 6-month extension to Study 3. In the extension phase, all 29 patients had withdrawn from imiglucerase and received open-label Zavesca 100 mg three times daily monotherapy. At Month 12, the results showed non-significant decreases in platelet counts from baseline in all the treatment groups (by original randomization). There was a significant decrease in platelet counts from Month 6 to Month 12 in the group originally randomized to treatment with imiglucerase, and a continued decrease in platelet counts in the group originally randomized to Zavesca alone. There were no significant changes in any treatment group for liver volume, spleen volume, or hemoglobin concentration (See Tables 7-10).
- Liver volume results from Study 3 and extension are summarized in Table 7:
- Patients with platelet counts above 150 × 109/L at baseline who were randomized to Zavesca treatment had significant decreases in platelet counts at Month 12.
How Supplied
- Zavesca® is supplied in hard gelatin capsules containing 100 mg miglustat. Zavesca® 100 mg capsules are white opaque with "OGT 918" printed in black on the cap and "100" printed in black on the body.
- Zavesca® 100 mg capsules are packed in blister cards. Five blister cards of 18 capsules are supplied in each carton.
- NDC 66215-201-90: carton containing 90 capsules.
- NDC 66215-201-18: blister card containing 18 capsules
- Storage: Store at 20°C to 25°C (68° to 77°F). Brief exposure to 15°C to 30°C (59°F to 86°F) permitted.
Storage
There is limited information regarding Miglustat Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Miglustat |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Miglustat |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise patients that the most common serious adverse reactions reported with Zavesca are peripheral neuropathy. Advise patients to promptly report any numbness, tingling, pain, or burning in the hands and feet.
- Advise patients that other adverse reactions include tremor and reductions in platelet counts. Advise patients to promptly report the development of tremor or worsening in an existing tremor.
- Advise patients that other serious adverse reactions include diarrhea and weight loss. Advise patients to adhere to dietary instructions.
- Advise patients to take the next Zavesca capsule at the next scheduled time if a dose is missed.
- Inform patients of the potential risks and benefits of Zavesca and of alternative modes of therapy.
Precautions with Alcohol
- Alcohol-Miglustat interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ZAVESCA®[1]
Look-Alike Drug Names
- Zavesca (miglustat)® — Zavesca (escitalopram®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "ZAVESCA- miglustat capsule".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Miglustat
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Miglustat |Label Name=Miglustat12.png
}}
{{#subobject:
|Label Page=Miglustat |Label Name=Miglustat13.png
}}