Tipranavir clinical pharmacology
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ahmed Zaghw, M.D. [2]
CLINICAL PHARMACOLOGY
Mechanism of Action
Tipranavir is an antiviral drug.
Pharmacodynamics
ECG Evaluation The effect of APTIVUS/ritonavir on the QTcF interval was measured in a study in which 81 healthy subjects received the following treatments twice daily for 2.5 days: APTIVUS/ritonavir (500/200 mg), APTIVUS/ritonavir at a supra-therapeutic dose (750/200 mg), and placebo/ritonavir (-/200 mg). After baseline and placebo adjustment, the maximum mean QTcF change was 3.2 ms (1-sided 95% Upper CI: 5.6 ms) for the 500/200 mg dose and 8.3 ms (1-sided 95% Upper CI: 10.9 ms) for the supra-therapeutic 750/200 mg dose.
Antiviral Activity in vivo The median Inhibitory Quotient (IQ) determined from 264 treatment-experienced adult patients was about 80 (inter-quartile range: 31-226), from the controlled clinical trials 1182.12 and 1182.48. The IQ is defined as the tipranavir trough concentration divided by the viral EC50 value, corrected for protein binding. There was a relationship between the proportion of patients with a ≥1 log10 reduction of viral load from baseline at week 48 and their IQ value. Among the 198 patients receiving APTIVUS/ritonavir with no new enfuvirtide use (e.g., new enfuvirtide, defined as initiation of enfuvirtide for the first time), the response rate was 23% in those with an IQ value <80 and 59% in those with an IQ value ≥80. Among the 66 patients receiving APTIVUS/ritonavir with new enfuvirtide, the response rates in patients with an IQ value <80 versus those with an IQ value ≥80 were 55% and 71%, respectively. These IQ groups are derived from a select population and are not meant to represent clinical breakpoints.
Pharmacokinetics
In order to achieve effective tipranavir plasma concentrations and a twice-daily dosing regimen, co-administration of APTIVUS with ritonavir is essential. Ritonavir inhibits hepatic cytochrome P450 3A (CYP 3A), the intestinal P-gp efflux pump and possibly intestinal CYP 3A. In a dose-ranging evaluation in 113 HIV-1 negative male and female volunteers, there was a 29-fold increase in the geometric mean morning steady-state trough plasma concentrations of tipranavir following APTIVUS co-administered with low-dose ritonavir (500/200 mg twice daily) compared to APTIVUS 500 mg twice daily without ritonavir. In adults the mean systemic ritonavir concentration when 200 mg of ritonavir was given with 500 mg of APTIVUS was similar to the concentrations observed when 100 mg was given with the other protease inhibitors.
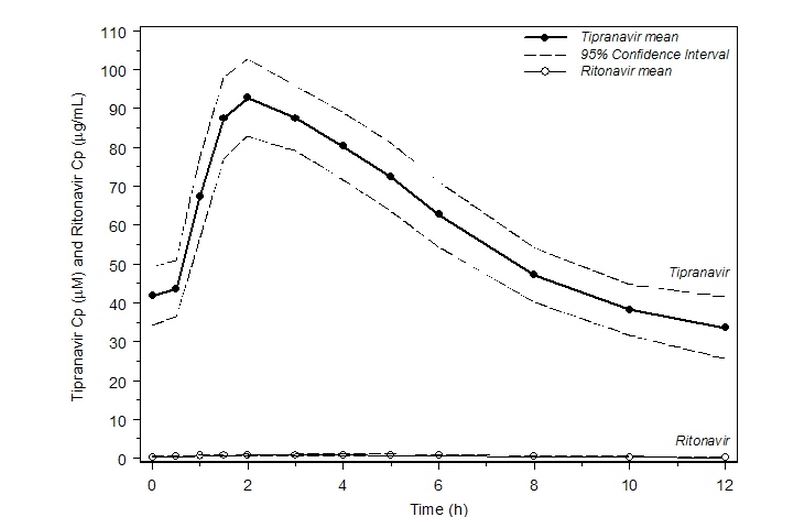
Figure 1 displays mean plasma concentrations of tipranavir and ritonavir at steady state for 30 HIV-1 infected adult patients dosed with 500/200 mg tipranavir/ritonavir for 14 days.
Figure 1 Mean Steady State Tipranavir Plasma Concentrations (95% CI) with Ritonavir Co-administration (tipranavir/ritonavir 500/200 mg BID)[1]
|
References
Adapted from the FDA Package Insert.