Rickettsia rickettsii
|
Rocky Mountain spotted fever Microchapters |
|
Differentiating Rocky Mountain spotted fever from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Rickettsia rickettsii On the Web |
|
American Roentgen Ray Society Images of Rickettsia rickettsii |
|
Directions to Hospitals Treating Rocky Mountain spotted fever |
| Rickettsia rickettsii | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
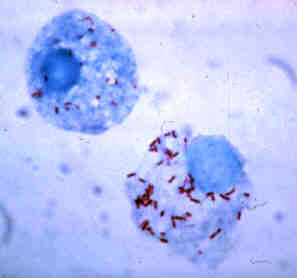 | ||||||||||||||||
| Scientific classification | ||||||||||||||||
| ||||||||||||||||
| Binomial name | ||||||||||||||||
| Rickettsia rickettsii Brumpt, 1922 |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Ilan Dock, B.S.
Overview
Rickettsia rickettsii (abbreviated as R. rickettsii) is a unicellular, Gram-negative coccobacillus (plural coccobacilli) that is native to the New World. It belongs to the spotted fever group (SFG) of Rickettsia and is most commonly known as the causative agent of Rocky Mountain spotted fever (RMSF). By nature, R. rickettsii is an obligate intracellular parasite that survive by an endosymbiotic relationship with other cells. [1]
R. rickettsii is a non-motile, non-spore forming aerobic organism. Cells are typically 0.3–0.5 × 0.8–2.0 μm in size. They lack a distinct nucleus and membrane bound organelles. Their outer membrane is composed mostly of lipopolysaccharides.
RMSF is transmitted by the bite of an infected tick while feeding on warm-blooded animals, including humans. Humans are considered to be accidental hosts in the Rickettsia–tick life cycle and are not required to maintain the rickettsiae in nature.[2]
History
- Rocky Mountain spotted fever first emerged in the Idaho Valley in 1896. At that time, not much information was known about the disease; it was originally called Black Measles because patients had a characteristic spotted rash appearance throughout their body. [3]
- Howard Ricketts (1871–1910) was an American pathologist and infectious disease researcher who was the first to identify and study the organism that causes Rocky Mountain spotted fever. He received his undergraduate degree in zoology from the University of Nebraska and his medical degree from Northwestern University School of Medicine.
- Ricketts completed his internship at Cook County Hospital in Chicago, IL, followed by a fellowship in pathology and cutaneous diseases at Rush Medical College.
- In 1902, Ricketts became the associate professor of pathology at the University of Chicago. The trademark rash, which first appeared in the Idaho Valley, now began to slowly emerge in the Bitterroot Valley region, a highly influential area in western Montana and had an 80–90% mortality rate. [4]
- During his tenure as associate professor, Ricketts was funded and recruited by the University of Chicago, the State of Montana, and the American Medical Association to conduct research on Rocky Mountain spotted fever.
- Ricketts research entailed interviewing victims of the disease and collecting and studying infected animals. He was also known to inject himself with pathogens to measure its effects.
- Days after isolating the organism that he believed to cause typhus, he died. It was speculated that his death was likely caused from an insect bite. [3]
- S. Burt Wolbach is credited for the first detailed description of the etiologic agent in 1919.
- He clearly recognized it as an intracellular bacterium which was seen most frequently in endothelial cells.
- Wolbach was struck by the fact that in the tick, and also in mammalian cells, the microorganism was intranuclear.
- The nucleus was often completely filled with minute particles and often was distended. Although Wolbach recognized its similarity to the agent of typhus and tsutsugamushi fever (scrub typhus), he did not regard the designation 'rickettsia' as appropriate. He proposed the name *Dermacentroxenus rickettsi. Dr. Emile Brumpt felt that the etiologic agent of RMSF, despite some uncertainty about its properties, belonged in the genus Rickettsia and in 1922 proposed the name Rickettsia rickettsii.[3]
Taxonomy
| Classifications | |
|---|---|
| Domain | Bacteria |
| Kingdom | Prokaryote |
| Phylum | Proteobacteria |
| Class | Alpha Protobacteria |
| Order | Rickettsiales |
| Family | Rickettsiaceae |
| Genus | Rickettsia |
| Species | Rickettsii |
Pathogen life cycle
- The life cycle of Rickettsia rickettsii is considered to be a complex one.
- Survival is dependent on both an invertebrate vector, (the hard tick- Family Ixodidae) and a vertebrate host (including mice, dogs, rabbits).
- Humans are considered to be accidental vectors and are not essential in the rickettsial cycle.
- In addition, a sequence of events occur between both hosts in the successful transmission of rickettsial disease.
- (Rickettsia rickettsii mostly affects canines and humans.) [6]
Transmission in arthropod vectors
- Three arthropod vectors in the United States have been identified in transmitting R. rickettsii to humans, causing the potentially fatal disease Rocky Mountain Spotted Fever. The American dog tick (Dermacentor variabilis), the Rocky Mountain Wood Tick (Dermacentor andersoni), and the Brown dog tick (Rhipicephalus sanguineus) can acquire Rickettsia rickettsii in a number of different ways.
- First, an uninfected tick can become infected when feeding on the blood of an infected mammalian host in the larval or nymph stages, a mode of transmission called transstadial transmission. Once a tick becomes infected with this pathogen, they are infected for life. Both the American dog tick and the Rocky Mountain wood tick serve as long-term reservoirs for Rickettsia rickettsii, in which the organism resides in the tick posterior diverticulae of the midgut, the small intestine and the ovaries.
- Due to its confinement in the midgut and small intestine, it is possible for mammals, including humans, to contract rickettsial disease from open skin/wound contact with the feces of the organism. In addition, an infected male tick can transmit the organism to an uninfected female during mating. Once infected, the female tick can transmit the infection to her offspring, in a process known as transovarial transmission. [7]
Transmission in mammals
- An uninfected mammal can become infected with Rickettsia rickettsii when eating food that contains the feces of the infected tick. They can also be infected through the bite of an infected tick.
- Humans acquire Rickettsia rickettsii infection from infected vectors. After getting bitten by an infected tick, rickettsiae are transmitted to the bloodstream by tick salivary secretions or, as mentioned previously, through contamination of broken skin by infected vector feces.
- All these modes of transmission ensures the survival of Rickettsia in nature.
- R. rickettsii have evolved a number of strategical mechanisms or virulence factors that allow them to evade the host immune system and successfully infect the host. [7]
Virulence
- R. rickettsii invades the endothelial cells that line the blood vessels. Endothelial cells are not phagocytic in nature; however, after attachment to the host cell surface, the pathogen causes changes in the host cell cytoskeleton that induces phagocytosis. They are able to avoid lysosomal fusion and oxidative burst by escaping from the phagosome into the cytoplasm where they multiply and spread.
- Over the years, different virulence factors have been identified in R. rickettsii.
OmpA and OmpB
- OmpA (rOmp) and Omp B (rOmp) have been identified as rickettsial outer surface proteins and are implicated in adherence of the bacterium to the host cell. The genes that encode these two surface proteins are designated as ompA and ompB, respectively.
- rOmp B is the predominant surface membrane protein in R. rickettsii; Policastro et al., identified the rOmpA to rOmpB ratio to be 1:9 (1994).[8] While the surface proteins of the bacterium have been identified, the host cell protein receptor(s) have not.
T4SS
- Entry into the host cell is mediated by a Type 4 secretion system' (T4SS) which is found in all Rickettsiae. The organization of the T4SS apparatus is a rather elaborate one; it is a tunnel-shaped structure that is embedded in the bacterial inner membrane and extends to the outer membrane. At least 12 or more proteins help form the tunnel-like apparatus.
- Once adherence to the host cell is established, the T4SS of Rickettsiae recruits substrates to the bottom of the apparatus, activating the complex via an ATP-dependent process that results in the direct transfer of the bacterium's DNA and other proteins into the host cell.
Phospholipase A2
- Invasion of the host endothelial cell immediately triggers phagocytosis, where the rickettsiae escape from the phagosome and into the cytosol where replication takes place. Although the escape from the phagosome is not well understood, it is thought to be mediated by phospholipase A2 activity.
Actin polymerization
- In the cytosol, the virulence factor Sca2 (Surface Cell Antigen 2) and the protein RickA form an actin tail that provides motility.
- RickA is thought to be responsible for directing the actin-based motility in R. rickettsii.
- RickA has been shown to activate the Arp2/3 complex in vitro, but "R. raoulti" expresses RickA and does not have acting-based motility.[9]
- The Sca2 virulence factor has been shown to be essential for actin tail formation in "R.rickettsii" Listeria. Comparisons of actin motility mechanisms appears to be independently evolved in Listeria, Shigella, and Rickettsia.
- The actin tail in R. rickettsii is both longer and provides a straighter trajectory due the production of linear actin filaments.[10]
- The actin-based motility of R. rickettsii allows swift, unidirectional movement[citation needed] across the cytoplasm into adjacent cells, promoting cell to cell spread.
- Endothelial cell damage caused by R. rickettsii can lead to end organ failure, DIC, and even death.
In vivo versus in vitro studies
- In vivo studies reveal that Rickettsia rickettsii invade endothelial lining of small to medium vessels in the human host, causing vascular permeability. When tested in vitro, it is shown that the bacterium infects every kind of cell of the mammalian host.
Epidemiology
- The name Rocky Mountain spotted fever is somewhat of a misnomer. Cases of Rocky Mountain spotted fever have been reported in every continent except Antarctica, and in every state in the U.S. except for Alaska, and Hawaii.
- Approximately 90% of all infections occur within the months of April to September, the time period in which adult and nymphal ticks are the highest. The areas of the U.S. with the greatest reported cases of RMSF are the mid to south Atlantic states, including DE, MD, DC, VA, WV, NC, SC.
- It is estimated that approximately 1200 or more new cases of RMSF will present on a yearly basis. [11]
References
- ↑ 1.0 1.1 Dantas-Torres, Filipe. Lancet Infect Disease 2007;7:724-32. Department of Immunology, Center of Research Aggeu Magalhaes, Oswaldo Cruz Foundation. Recife Pernambuco, Brazil. Volume 7, November 2007. Accessed on January 11, 2016
- ↑ Rocky Moutnain Spotted Fever. Department of Health. Information for a Healthy New York. https://www.health.ny.gov/diseases/communicable/rocky_mountain_spotted_fever/fact_sheet.htm Accessed on January 11, 2016
- ↑ 3.0 3.1 3.2 Spencer R.R., Parker R.R. Studies on Rocky Mountain spotted fever. U.S. G.P.O. Washington, 1930. 16141346. Hygienic Laboratory Bulletin. V-154. http://books.google.com.au/books?id=6C9DAAAAYAAJ}}
- ↑ Overview. Rocky Mountain Spotted Fever. Centers for Disease Control http://www.cdc.gov/ncidod/dvrd/rmsf/overview.htm Accessed June 24, 2009
- ↑ The Cause of Rocky Mountain Spotted Fever. Rickettsia Ricketsii. http://bioweb.uwlax.edu/bio203/s2008/gibson_chel/Classification.htm Accessed January 11, 2016.
- ↑ Walker, David H. Medical Microbiology 4th Edition. Chapter 38. Rickettsiae. (1996). http://www.ncbi.nlm.nih.gov/books/NBK7624/#A2139 Accessed on January 7, 2016
- ↑ 7.0 7.1 Azad, F. Abdu; Beard, B. Charles. Ricketssial Pathogens and their Arthropod Vectors (1998). http://wwwnc.cdc.gov/eid/article/4/2/pdfs/98-0205.pdf Accessed on January 7, 2016
- ↑ Policastro PF, Hackstadt T (November 1994). "Differential activity of Rickettsia rickettsii opmA and ompB promoter regions in a heterologous reporter gene system" (PDF). Microbiology (Reading, Engl.). 140 (11): 2941–9. doi:10.1099/13500872-140-11-2941. PMID 7812435.
- ↑ Kleba, Betsy; Tina R. Clark; Erika I. Lutter; Damon W. Ellison; Ted Hackstadt (March 1, 2010). "Disruption of the Rickettsia rickettsii Sca2 Autotransporter Inhibits Actin-Based Motility". Infection and Immunity. 78 (5): 2240–7. doi:10.1128/IAI.00100-10. PMC 2863521. PMID 20194597. Retrieved March 9, 2014.
- ↑ Goldberg, Marcia (December 2001). "Actin-Based Motility of Intracellular Microbial Pathogens". MICROBIOLOGY AND MOLECULAR BIOLOGY REVIEWS. 65 (4): 31. doi:10.1128/MMBR.65.4.595-626.2001. PMC 99042. PMID 11729265. Retrieved March 9, 2014.
- ↑ Rocky Mountain Spotted Fever Statistics. Centers for Disease Control and Prevention (2015). http://www.cdc.gov/rmsf/stats/ Accessed on December 30, 2015
- Garrity, George; Brenner, Don J.; Staley, James T.; Krieg, Noel R.; Boone, David R.; Vos, Paul De; Goodfellow, Michael; Rainey, Fred A.; Schleifer, Karl-Heinz (2006). "Order II. Rickettsiales Gieszczkiewicz 1939..". Bergey's Manual® of Systematic Bacteriology: Volume Two: The Proteobacteria (Part C). Springer. pp. 96–. ISBN 978-0-387-29298-4.
- Weiss, K. (1988). "The Role of Rickettsioses in History". In Walker, David H. Biology of Rickettsial Diseases. CRC Press. pp. 2–14. ISBN 978-0-8493-4382-7.
- Weiss, E. (1988). "History of Rickettsiology". Biology of Rickettsial Diseases. pp. 15–32.
- Wilson, Brenda A.; Salyers, Abigail A.; Whitt, Dixie D.; Winkler, Malcolm E. (2011). Bacterial Pathogenesis: A Molecular Approach (3rd ed.). Amer Society for Microbiology. ISBN 978-1-55581-418-2.
External links
- "Rickettsia rickettsii genomes and related information". PATRIC Bioinformatics Resource Center. NIAID. External link in
|publisher=, |work=(help) - "Rickettsia rickettsii: The Cause of Rocky Mountain Spotted Fever". Multiple Organisms: Organismal Biology. University of Wisconsin-La Crosse. 2007.
- Todar, Kenneth (2008–2012). "Rickettsial Diseases, including Typhus and Rocky Mountain Spotted Fever". Todar's Online Textbook of Bacteriology.
- "Rocky Mountain Spotted Fever (RMSF)". Centers for Disease Control and Prevention. 21 November 2013.