Ticlopidine adverse reactions: Difference between revisions
Ahmed Zaghw (talk | contribs) |
Ahmed Zaghw (talk | contribs) No edit summary |
||
| Line 27: | Line 27: | ||
===Rash=== | ===Rash=== | ||
Ticlopidine has been associated with a maculopapular or urticarial [[rash]] (often with pruritus). Rash usually occurs within 3 months of initiation of therapy with a mean onset time of 11 days. If drug is discontinued, recovery occurs within several days. Many rashes do not recur on drug rechallenge. There have been rare reports of severe [[rashes]], including [[Stevens-Johnson syndrome]], [[erythema multiforme]] and [[exfoliative dermatitis]]. | Ticlopidine has been associated with a maculopapular or urticarial [[rash]] (often with pruritus). Rash usually occurs within 3 months of initiation of therapy with a mean onset time of 11 days. If drug is discontinued, recovery occurs within several days. Many rashes do not recur on drug rechallenge. There have been rare reports of severe [[rashes]], including [[Stevens-Johnson syndrome]], [[erythema multiforme]] and [[exfoliative dermatitis]]. | ||
==Less Frequent Adverse Reactions (Probably Related)== | ==Less Frequent Adverse Reactions (Probably Related)== | ||
Clinical adverse experiences occurring in 0.5% to 1.0% of stroke patients in controlled trials include: | Clinical adverse experiences occurring in 0.5% to 1.0% of stroke patients in controlled trials include: | ||
===Digestive System=== | ===Digestive System=== | ||
GI fullness | GI fullness | ||
===Skin and Appendages=== | ===Skin and Appendages=== | ||
[[Urticaria]] | [[Urticaria]] | ||
===Nervous System=== | ===Nervous System=== | ||
[[Headache]] | [[Headache]] | ||
===Body as a Whole=== | ===Body as a Whole=== | ||
[[Asthenia]], [[pain]] | [[Asthenia]], [[pain]] | ||
===Hemostatic System=== | ===Hemostatic System=== | ||
[[Epistaxis]] | [[Epistaxis]] | ||
===Special Senses=== | ===Special Senses=== | ||
[[Tinnitus]] | [[Tinnitus]] | ||
==Rare Relatively Serious and Potentially Fatal Events== | ==Rare Relatively Serious and Potentially Fatal Events== | ||
In addition, rarer, relatively serious and potentially fatal events associated with the use of [[ticlopidine]] have also been reported from postmarketing experience: [[Hemolytic anemia]] with [[reticulocytosis]], immune [[thrombocytopenia]], [[hepatitis]], hepatocellular jaundice, cholestatic [[jaundice]], hepatic necrosis, [[hepatic failure]], [[peptic ulcer]], [[renal failure]], [[nephrotic syndrome]], [[hyponatremia]], [[vasculitis]], [[sepsis]], allergic reactions (including [[angioedema]], [[Hypersensitivity pneumonitis overview|allergic pneumonitis]], and [[anaphylaxis]] ), [[systemic lupus]] (positive ANA), [[peripheral neuropathy]], [[serum sickness]], [[arthropathy]] and [[myositis]].<ref name="dailymed.nlm.nih.gov">{{Cite web | last = | first = | title = TICLOPIDINE HYDROCHLORIDE TABLET, FILM COATED [APOTEX CORP.] | url =http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=707127cb-cdcd-81b0-274d-3c11fefa6824 | publisher = | date = | accessdate = }}</ref> | In addition, rarer, relatively serious and potentially fatal events associated with the use of [[ticlopidine]] have also been reported from postmarketing experience: [[Hemolytic anemia]] with [[reticulocytosis]], immune [[thrombocytopenia]], [[hepatitis]], hepatocellular jaundice, cholestatic [[jaundice]], hepatic necrosis, [[hepatic failure]], [[peptic ulcer]], [[renal failure]], [[nephrotic syndrome]], [[hyponatremia]], [[vasculitis]], [[sepsis]], allergic reactions (including [[angioedema]], [[Hypersensitivity pneumonitis overview|allergic pneumonitis]], and [[anaphylaxis]] ), [[systemic lupus]] (positive ANA), [[peripheral neuropathy]], [[serum sickness]], [[arthropathy]] and [[myositis]].<ref name="dailymed.nlm.nih.gov">{{Cite web | last = | first = | title = TICLOPIDINE HYDROCHLORIDE TABLET, FILM COATED [APOTEX CORP.] | url =http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=707127cb-cdcd-81b0-274d-3c11fefa6824 | publisher = | date = | accessdate = }}</ref> | ||
Revision as of 16:53, 6 February 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ahmed Zaghw, M.D. [2]
Adverse Reactions
Adverse reactions in stroke patients were relatively frequent with over 50% of patients reporting at least one. Most (30% to 40%) involved the gastrointestinal tract. Most adverse effects are mild, but 21% of patients discontinued therapy because of an adverse event, principally diarrhea, rash, nausea, vomiting, GI pain and neutropenia. Most adverse effects occur early in the course of treatment, but a new onset of adverse effects can occur after several months.
The incidence rates of adverse events listed in the following table were derived from multicenter, controlled clinical trials in stroke patients described above comparing ticlopidine, placebo and aspirin over study periods of up to 5.8 years. Adverse events considered by the investigator to be probably drug-related that occurred in at least 1% of patients treated with ticlopidine are shown in the following table:
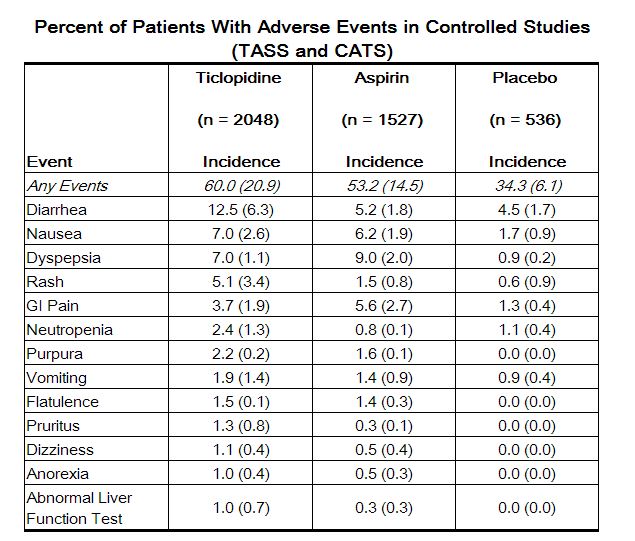 |
Incidence of discontinuation, regardless of relationship to therapy, is shown in parentheses.
Hematological
Neutropenia/thrombocytopenia, TTP, aplastic anemia (see BOXED WARNING and WARNINGS), leukemia, agranulocytosis, eosinophilia, pancytopenia, thrombocytosis and bone-marrow depression have been reported.
Gastrointestinal
Ticlopidine therapy has been associated with a variety of gastrointestinal complaints including diarrhea and nausea. The majority of cases are mild, but about 13% of patients discontinued therapy because of these. They usually occur within 3 months of initiation of therapy and typically are resolved within 1 to 2 weeks without discontinuation of therapy. If the effect is severe or persistent, therapy should be discontinued. In some cases of severe or bloody diarrhea, colitis was later diagnosed.
Hemorrhagic
Ticlopidine has been associated with increased bleeding, spontaneous posttraumatic bleeding and perioperative bleeding including, but not limited to, gastrointestinal bleeding. It has also been associated with a number of bleeding complications such as ecchymosis, epistaxis, hematuria and conjunctival hemorrhage.
Intracerebral bleeding was rare in clinical trials in stroke patients with Ticlopidine, with an incidence no greater than that seen with comparator agents (ticlopidine 0.5%, aspirin 0.6%, placebo 0.75%). It has also been reported postmarketing.
Rash
Ticlopidine has been associated with a maculopapular or urticarial rash (often with pruritus). Rash usually occurs within 3 months of initiation of therapy with a mean onset time of 11 days. If drug is discontinued, recovery occurs within several days. Many rashes do not recur on drug rechallenge. There have been rare reports of severe rashes, including Stevens-Johnson syndrome, erythema multiforme and exfoliative dermatitis.
Less Frequent Adverse Reactions (Probably Related)
Clinical adverse experiences occurring in 0.5% to 1.0% of stroke patients in controlled trials include:
Digestive System
GI fullness
Skin and Appendages
Nervous System
Body as a Whole
Hemostatic System
Special Senses
Rare Relatively Serious and Potentially Fatal Events
In addition, rarer, relatively serious and potentially fatal events associated with the use of ticlopidine have also been reported from postmarketing experience: Hemolytic anemia with reticulocytosis, immune thrombocytopenia, hepatitis, hepatocellular jaundice, cholestatic jaundice, hepatic necrosis, hepatic failure, peptic ulcer, renal failure, nephrotic syndrome, hyponatremia, vasculitis, sepsis, allergic reactions (including angioedema, allergic pneumonitis, and anaphylaxis ), systemic lupus (positive ANA), peripheral neuropathy, serum sickness, arthropathy and myositis.[1]
References
Adapted from the FDA Package Insert.