Atovaquone proguanil indications and usage: Difference between revisions
| Line 51: | Line 51: | ||
====Renal Impairment==== | ====Renal Impairment==== | ||
Do not use Atovaquone proguanil for malaria prophylaxis in patients with severe renal impairment (creatinine clearance <30 mL/min) [see Contraindications | Do not use Atovaquone proguanil for malaria prophylaxis in patients with severe renal impairment (creatinine clearance <30 mL/min) [see Contraindications ]. Use with caution for the treatment of malaria in patients with severe renal impairment, only if the benefits of the 3-day treatment regimen outweigh the potential risks associated with increased drug exposure. No dosage adjustments are needed in patients with mild (creatinine clearance 50 to 80 mL/min) or moderate (creatinine clearance 30 to 50 mL/min) renal impairment. [See Clinical Pharmacology ]<ref name="dailymed.nlm.nih.gov">{{Cite web | last = | first = | title = MALARONE (ATOVAQUONE AND PROGUANIL HYDROCHLORIDE) TABLET, FILM COATED [GLAXOSMITHKLINE LLC] | url = http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=fc22e8d8-3bfb-4f70-a599-dd81262a4887 | publisher = | date = | accessdate = }}</ref> | ||
==References== | ==References== | ||
Revision as of 04:51, 7 January 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Chetan Lokhande, M.B.B.S [2]
Indications And Usage
Prevention of Malaria
Atovaquone proguanil® is indicated for the prophylaxis of Plasmodium falciparum malaria, including in areas where chloroquine resistance has been reported.
Treatment of Malaria
Atovaquone proguanil is indicated for the treatment of acute, uncomplicated P. falciparum malaria. Atovaquone proguanil has been shown to be effective in regions where the drugs chloroquine, halofantrine, mefloquine, and amodiaquine may have unacceptable failure rates, presumably due to drug resistance.
Dosage And Administration
The daily dose should be taken at the same time each day with food or a milky drink. In the event of vomiting within 1 hour after dosing, a repeat dose should be taken.
Atovaquone proguanil may be crushed and mixed with condensed milk just prior to administration to patients who may have difficulty swallowing tablets.
Prevention of Malaria
Start prophylactic treatment with Atovaquone proguanil 1 or 2 days before entering a malaria‑endemic area and continue daily during the stay and for 7 days after return.
Adults
One Atovaquone proguanil Tablet (adult strength = 250 mg atovaquone/100 mg proguanil hydrochloride) per day.
Pediatric Patients
The dosage for prevention of malaria in pediatric patients is based upon body weight (Table 1).
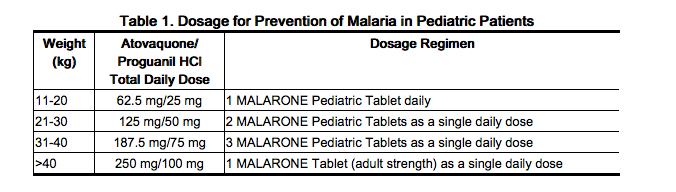 |
Treatment of Acute Malaria
Adults
Four Atovaquone proguanil Tablets (adult strength; total daily dose 1 g atovaquone/400 mg proguanil hydrochloride) as a single daily dose for 3 consecutive days.
Pediatric Patients
The dosage for treatment of acute malaria in pediatric patients is based upon body weight (Table 2).
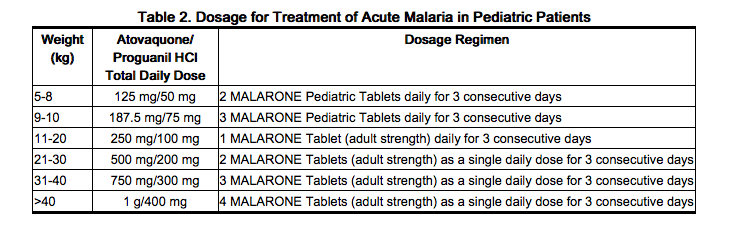 |
Renal Impairment
Do not use Atovaquone proguanil for malaria prophylaxis in patients with severe renal impairment (creatinine clearance <30 mL/min) [see Contraindications ]. Use with caution for the treatment of malaria in patients with severe renal impairment, only if the benefits of the 3-day treatment regimen outweigh the potential risks associated with increased drug exposure. No dosage adjustments are needed in patients with mild (creatinine clearance 50 to 80 mL/min) or moderate (creatinine clearance 30 to 50 mL/min) renal impairment. [See Clinical Pharmacology ][1]
References
Adapted from the FDA Package Insert.