Tularemia: Difference between revisions
No edit summary |
|||
| Line 154: | Line 154: | ||
== External links == | == External links == | ||
*[http://www.bt.cdc.gov/agent/tularemia/index.asp CDC Emergency Preparedness and Response index for tularemia] | *[http://www.bt.cdc.gov/agent/tularemia/index.asp CDC Emergency Preparedness and Response index for tularemia] | ||
== References == | == References == | ||
Revision as of 21:18, 10 December 2012
|
Tularemia Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Tularemia On the Web |
|
American Roentgen Ray Society Images of Tularemia |
For patient information click here
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Synonyms and keywords: Yatobyo; deer fly fever; Francis' disease; Francisella tularensis infection; Ohara disease; Pahvant Valley fever; rabbit fever
Overview
Tularemia is a serious infectious disease caused by the bacterium Francisella tularensis. The disease is endemic in North America, and parts of Europe and Asia. The primary vectors are ticks and deer flies, but the disease can also be spread through other arthropods. Animals such as rabbits, prairie dogs, hares and muskrats serve as reservoir hosts. The disease is named after Tulare County, California.
History
Franciscella tularensis was discovered in 1911 during an outburst of rabbit fever, when the disease killed a large number of ground squirrels in the area of Tulare Lake in California. Scientists determined that tularemia could be dangerous to humans: a human being may catch the infection after contacting an infected animal. The ailment soon became frequent with hunters, cooks and agricultural workers.[2]
Epidemiology and Demographics

- Tularemia occurs throughout much of North America and Eurasia. In the U.S., human cases have been reported from every state except Hawaii, with the majority occurring in south-central and western states.
- F. tularensis is found in widely diverse animal hosts and habitats and can be recovered from contaminated water, soil, and vegetation. A variety of small mammals, including voles, mice, water rats, squirrels, rabbits, and hares are natural reservoirs of infection. They acquire infection through tick, fly, and mosquito bites and by contact with contaminated environments. Epizootics with sometimes extensive die-offs of animal hosts may herald outbreaks of tularemia in humans.
- Humans can become incidentally infected through diverse environmental exposures: bites by infected arthropods; handling infectious animal tissues or fluids; direct contact with or ingestion of contaminated food, water, or soil; and inhalation of infective aerosols. Humans can develop severe and sometimes fatal illness, but do not transmit the disease to others.
- The high incidence of tularemia among males and among children aged <10 years might be associated with increased opportunity for exposure to infected ticks or animals, less use of personal protective measures against tick bites, or diagnostic or reporting bias. The high incidence among American Indians/Alaska Natives might be associated with their increased risk for exposure; outbreaks of tularemia have been reported on reservations in Montana and South Dakota, where a high prevalence of tularemia infection was found in ticks and dogs.
- Worldwide incidence of naturally occurring tularemia is unknown. It is likely that the disease is greatly under-recognized and under-reported. In the U.S., reported cases have dropped sharply from several thousand/year prior to 1950 to fewer than 200/year in the 1990s. Between 1985 and 1992, 1409 cases and 20 deaths were reported in the U.S., a case fatality rate of 1.4%. Most U.S. cases occur June–September, when arthropod-borne transmission is most common. Cases in winter most commonly occur among hunters and trappers who handle infected animal carcasses.
- Release in a densely populated area would be expected to result in an abrupt onset of large numbers of acute, nonspecific febrile illness beginning 3–5 days later (incubation range 1–14 days), with pleuropneumonitis developing in a significant proportion of cases during the ensuing days and weeks.[1] [2].
Risk Factors
In the United States, most persons with tularemia acquire the infection from arthropod bites, particularly tick bites, or from contact with infected mammals, particularly rabbits. In recent years, a seasonal increase in incidence has occurred only in the late spring and summer months, when arthropod bites are most common. Outbreaks of tularemia in the United States have been associated with muskrat handling, tick bites, deerfly bites, and lawn mowing or cutting brush. Sporadic cases in the United States have been associated with contaminated drinking water and various laboratory exposures. Outbreaks of pneumonic tularemia, particularly in low-incidence areas, should prompt consideration of bioterrorism.[3]
Pathophysiology & Etiology
Tularemia is caused by the bacterium Francisella tularensis found in animals (especially rodents, rabbits, and hares). Francisella tularensis (F. tularensis) is a tiny, pleomorphic, nonmotile, gram-negative, facultative intracellular coccobacillus (0.2 to 0.5 μm by 0.7 to 1.0 μm). It is a fastidious organism and may require cysteine supplementation for good growth on general laboratory media.
- F. tularensis can infect humans through the skin, mucous membranes, gastrointestinal tract, and lungs. It is a facultative intracellular bacterium that multiplies within macrophages. The major target organs are the lymph nodes, lungs and pleura, spleen, liver, and kidney. Untreated, bacilli inoculated into skin or mucous membranes multiply, spread to regional lymph nodes and further multiply, and then may disseminate to organs throughout the body.
- Bacteremia may be common in the early phase of infection. The initial tissue reaction to infection is a focal, intensely suppurative necrosis consisting largely of accumulations of polymorphonuclear leukocytes, followed by invasion of macrophages, epithelioid cells, and lymphocytes.
- Suppurative lesions become granulomatous, and histopathological examination of the granulomas shows a central necrotic, sometimes caseating, zone surrounded by a layer of epithelioid cells, multinucleated giant cells, and fibroblasts in a radial arrangement, typical of other granulomatous conditions such as tuberculosis and sarcoidosis.
- Humans with inhalational exposures also develop hemorrhagic inflammation of the airways early in the course of illness, which may progress to bronchopneumonia. Histopathological examination of the lungs shows alveolar spaces filled with an exudate of mononuclear cells. Pleuritis with adhesions and effusion and hilar lymphadenopathy are common in radiological and pathological findings.
- Primary clinical forms vary in severity and presentation according to virulence of the infecting organism, dose, and site of inoculum.
- The onset of tularemia is usually abrupt, with fever (38oC–40oC), headache, chills and rigors, generalized body aches (often prominent in the low back), coryza, and sore throat. A pulse-temperature dissociation has been noted in as many as 42% of patients. A dry or slightly productive cough and substernal pain or tightness frequently occur with or without objective signs of pneumonia, such as purulent sputum, dyspnea, tachypnea, pleuritic pain, or hemoptysis. Nausea, vomiting, and diarrhea may occur.
- Sweats, fever, chills, progressive weakness, malaise, anorexia, and weight loss characterize the continuing illness.
- In general, tularemia would be expected to have a slower progression of illness and a lower case-fatality rate than either inhalational plague or anthrax. Milder forms of inhalational tularemia would be indistinguishable from Q fever; another potential bioterrorism agent; establishing a diagnosis of either would be problematic without reference laboratory testing.[4][5][6]
Mechanism of infection
Francisella tularensis is one of the most infective bacteria known; fewer than ten organisms can cause disease leading to severe illness. The bacteria penetrate into the body through damaged skin and mucous membranes, or through inhalation. Humans are most often infected by tick bite or through handling an infected animal. Ingesting infected water, soil, or food can also cause infection. Tularemia can also be acquired by inhalation; hunters are at a higher risk for this disease because of the potential of inhaling the bacteria during the skinning process. It has been contracted from inhaling particles from an infected rabbit ground up in a lawnmower (see below). Tularemia is not spread directly from person to person.
Francisella tularensis is an intracellular bacterium, meaning that it is able to live as a parasite within host cells. It primarily infects macrophages, a type of white blood cell. It is thus able to evade the immune system. The course of disease involves spread of the organism to multiple organ systems, including the lungs, liver, spleen, and lymphatic system. The course of disease is similar regardless of the route of exposure. Mortality in untreated (pre-antibiotic-era) patients has been as high as 50% in the pneumoniac and typhoidal forms of the disease, which however account for less than 10% of cases.[7] Overall mortality was 7% for untreated cases, and the disease responds well to antibiotics with a fatality rate of about 2%. The exact cause of death is unclear, but it is thought be a combination of multiple organ system failures.
Symptoms
- sudden fever
- chills
- headaches
- diarrhea
- muscle aches
- joint pain
- dry cough
- progressive weakness
People can also catch pneumonia and develop chest pain, bloody sputum and can have trouble breathing and even sometimes stop breathing.
Other symptoms of tularemia depend on how a person was exposed to the tularemia bacteria. These symptoms can include ulcers on the skin or mouth, swollen and painful lymph glands, swollen and painful eyes, and a sore throat.
Diagnosis
- Rapid diagnostic testing for tularemia is not widely available. Physicians who suspect inhalational tularemia in patients presenting with atypical pneumonia, pleuritis, and hilar lymphadenopathy should promptly collect specimens of respiratory secretions and blood and alert the laboratory to the need for special diagnostic and safety procedures.
- F. tularensis may be identified through direct examination of secretions, exudates, or biopsy specimens using Gram stain, direct fluorescent antibody, or immunohistochemical stains. Microscopic demonstration of F. tularensis using fluorescent-labeled antibodies is a rapid diagnostic procedure performed in designated reference laboratories in the National Public Health Laboratory Network; test results can be available within several hours of receiving the specimens, if the laboratory is alerted and prepared.
- Growth of F. tularensis in culture is the definitive means of confirming the diagnosis of tularemia. It can be grown from pharyngeal washings, sputum specimens, and even fasting gastric aspirates in a high proportion of patients with inhalational tularemia. It is only occasionally isolated from blood.
Clinical manifestations
Depending on the site of infection, tularemia has six characteristic clinical syndromes: ulceroglandular, glandular, oropharyngeal, pneumonic, oculoglandular, and typhoidal.[8]
The disease has a very rapid onset, with headache, fatigue, dizziness, muscle pains, loss of appetite and nausea. Face and eyes redden and become inflamed. Inflammation spreads to the lymph nodes, which enlarge and may suppurate (mimicking bubonic plague). Lymph node involvement is accompanied by a high fever. Death may result.[3]
Laboratory Findings


- Stains and smears: Gram stain
- Procedure: Perform Gram stain procedure/quality control per standard laboratory protocol.
- Characteristics: Staining of F. tularensis often reveals the presence of tiny, 0.2-0.5-μm X 0.7-1.0 μm, pleomorphic, poorly staining, gram-negative coccobacilli seen mostly as single cells. The gram stain interpretation may be difficult because the cells are minute and faintly staining. F. tularensis cells are smaller than Haemophilus influenzae. Bipolar staining is not a distinctive feature of F. tularensis cells.
- Additional work: Another smear may be prepared for referral to your state public health laboratory.
- Cultures
- Established inoculation and plating procedures are used. For tissues, established laboratory procedure is used to inoculate media (e.g., grind, touch-preparation, or a sterile wood stick). Plates are taped shut in 2 places to prevent inadvertent opening (alternate to taping is acceptable).
- Incubation of cultures.
- Temperature: 35-37°C
- Atmosphere: Ambient, use of 5% CO2 is acceptable.
- Length of incubation: primary plates are held for 5 days. If it is known that patient has been treated with bacteriostatic antibiotics, then plates are held for up to 7 days to allow bacteria recovery time.
- Characteristics: F. tularensis grows in commercial blood culture media. These organisms require cysteine supplementation; therefore, F. tularensis may at first grow on SBA, but upon subsequent passage will fail to grow on standard SBA. On cysteine supplemented agar plates, it is a gray-white, opaque colony, usually too small to be seen at 24 h on most general media such as CA, TM, and BCYE. After incubation for 48 h or more, colonies are about 1-2 mm in diameter, white to grey to bluish-grey, opaque, flat, with an entire edge, smooth, and have a shiny surface. F. tularensis will not grow on MacConkey or EMB plates.
- Biochemical reactions/tests
- Procedure: Use established laboratory procedures for catalase, oxidase,beta-lactamase, XV (or satellite), and urease tests.
- Interpretation: According to established laboratory practice.
- Additional notes: Commercial biochemical identification systems are not recommended at this stage.
Treatment
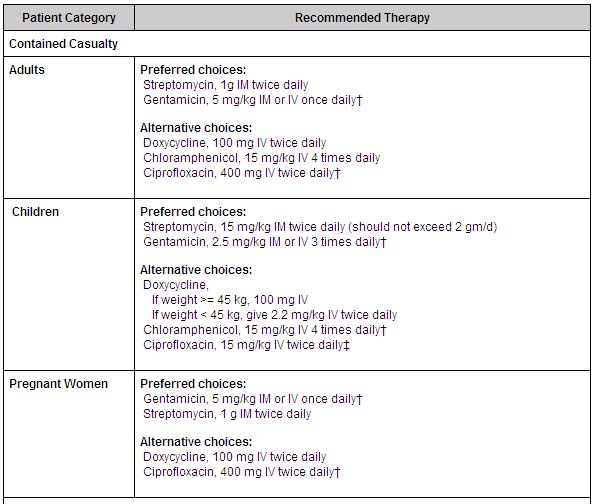
The drug of choice is Streptomycin.[9] Tularemia may also be treated with gentamicin, tetracycline, chloramphenicol or fluoroquinolones.
- In a contained casualty setting, where individual patient management is possible, the working group recommends parenteral antimicrobial therapy. Streptomycin is the drug of choice. Gentamicin, which is more widely available and can be used intravenously, is an acceptable alternative. Treatment with aminoglycosides should be continued for 10 days. Tetracyclines and chloramphenicol are also used, but relapses and primary treatment failures occur at a higher rate with these bacteriostatic agents than with aminogylcosides, and they should be given for at least 14 days to avoid relapse. Both streptomycin and gentamicin are recommended as first-line treatment of tularemia in children.
- In a mass casualty setting, doxycycline and ciprofloxacin, administered orally, are the preferred choices for treatment of both adults and children. As described in the table below, 'Treatment with ciprofloxacin should be continued for 10 days; treatment with doxycycline should be continued for 14-21 days.'
- Since it is unknown whether drug-resistant organisms might be used in a bioterrorist event, antimicrobial susceptibility testing of isolates should be conducted quickly and treatments altered according to test results and clinical responses.
- Antibiotics for treating patients infected with tularemia in a bioterrorist event are included in the national pharmaceutical stockpile maintained by CDC, as are ventilators and other emergency equipment.[10][11]
Primary Prevention
In the United States, a live attenuated vaccine derived from avirulent F. tularensis biovar palaearctica (type B) has been used to protect laboratorians routinely working with the bacterium. Until recently, this vaccine was available as an investigational new drug. It is currently under review by the Food and Drug Administration.
Tularemia occurs naturally in many parts of the United States. Use insect repellent containing DEET on your skin, or treat clothing with repellent containing permethrin, to prevent insect bites. Wash your hands often, using soap and warm water, especially after handling animal carcasses. Be sure to cook your food thoroughly and that your water is from a safe source.
Note any change in the behavior of your pets (especially rodents, rabbits, and hares) or livestock, and consult a veterinarian if they develop unusual symptoms.[12][13]
Secondary Prevention
What is CDC Doing About Tularemia?
The CDC operates a national program for bioterrorism preparedness and response that incorporates a broad range of public health partnerships. Other things CDC is doing include:
- Stockpiling antibiotics to treat infected people
- Coordinating a nation-wide program where states share information about tularemia
- Creating new education tools and programs for health professionals, the public, and the media.
Tularemia as a biological weapon
The Centers for Disease Control and Prevention regard F. tularensis as a viable bioweapons agent for use by terrorists. The disease was used as a weapon by the Russians during World War II.[4] Practical research into using Tularemia as a bioweapon took place at Camp Detrick in the 1950s. It was viewed as an attractive agent because:
- it is easy to aerosolize
- it is highly infective; fewer than 10 bacteria are required to infect
- it is non-persistent and easy to decontaminate (unlike anthrax)
- it is highly incapacitating to infected persons
- it has low-lethality, which is useful where enemy soldiers are in proximity to non-combatants, eg civilians
No vaccine is available to the general public.[14] The best way to prevent tularemia infection is to wear rubber gloves when handling or skinning rodents or lagomorphs (as rabbits), avoid ingesting uncooked wild game and untreated water sources, and wearing long-sleeved clothes and using an insect repellant to prevent tick bites.
Documented outbreaks
In summer 2000, an outbreak of tularemia in Martha's Vineyard resulted in one fatality, and brought the interest of the CDC as a potential investigative ground for aerosolized Francisella tularensis. Over the following summers, Martha's Vineyard was identified as the only place in the world where documented cases of tularemia resulted from lawn mowing.[15] The research may prove valuable in preventing bioterrorism.
An outbreak of tularemia occurred in Kosovo in 1999-2000 [5].
In 2004, three researchers at Boston University Medical Center were accidentally infected with F. tularensis, after apparently failing to follow safety procedures.[16]
In 2005, small amounts of F. tularensis were detected in the Mall area of Washington, DC the morning after an anti-war demonstration on September 24, 2005. Biohazard sensors were triggered at six locations surrounding the Mall. To this date, no cases of tularemia infection have been reported as a result.[17]
In 2007, a lab of Boston University's Center for Advanced Biomedical Research, where F. tularensis were being kept for research, was evacuated after smoke set off alarms. An investigation has later determined that an electrical problem was the culprit, and no bacterial contamination was found.
In July 2007, an outbreak was reported in the Spanish autonomous region of Castile and León and traced to the plague of voles infesting the region.
Biological Warfare
By the late 1950's the US biological warfare program was focused mostly on tularemia as a biological agent. The Schu S4 strain was standardized as Agent UL for use in the M143 bursting spherical bomblet. It was a lethal biological with an anticipated fatality rate of 40 to 60 percent. The rate-of-action was around three days, with a duration-of-action of 1 to 3 weeks (treated) and 2 to 3 months (untreated) with frequent relapses. UL was streptomycin resistant. The aerobiological stability of UL was a major concern, being sensitive to sun light, and losing virulence over time after release.
The United States later changed the military symbol for UL to TT (wet-type) and ZZ (dry-type) in an effort to retain security on the identity of military biologicals. When the 425 strain was standardized as agent JT (an incapacitant rather than lethal agent), the Schu S4 strain's symbol was changed again to SR.
Acknowledgements
The content on this page was first contributed by: C. Michael Gibson, M.S., M.D.
List of contributors:
Pilar Almonacid
External links
References
- ↑ http://www.bt.cdc.gov/agent/tularemia/tularemia-biological-weapon-abstract.asp#2
- ↑ http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5109a1.htm
- ↑ http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5109a1.htm
- ↑ http://www.bt.cdc.gov/agent/tularemia/facts.asp
- ↑ http://www.asm.org/ASM/files/LEFTMARGINHEADERLIST/DOWNLOADFILENAME/0000000525/tularemiaprotocol%5B1%5D.pdf
- ↑ http://www.bt.cdc.gov/agent/tularemia/tularemia-biological-weapon-abstract.asp#2
- ↑ http://www.cidrap.umn.edu/cidrap/content/bt/tularemia/biofacts/tularemiafactsheet.html#_Overview_1
- ↑ Plourde PJ, Embree J, Friesen F, Lindsay G, Williams T (1992). "Glandular tularemia with typhoidal features in a Manitoba child". Can Med Assoc J. 146: 1953&ndash, 5.
- ↑ Enderlin G, Morales L, Jacobs RF, Cross JT (1994). "Streptomycin and alternative agents for the treatment of tularemia: review of the literature". Clin Infect Dis. 19: 42&ndash, 7.
- ↑ http://www.bt.cdc.gov/agent/tularemia/facts.asp
- ↑ http://www.bt.cdc.gov/agent/tularemia/tularemia-biological-weapon-abstract.asp#2
- ↑ http://www.bt.cdc.gov/agent/tularemia/tularemia-biological-weapon-abstract.asp#2
- ↑ http://www.bt.cdc.gov/agent/tularemia/facts.asp,
- ↑ http://www.niaid.nih.gov/factsheets/tularemia.htm
- ↑ Feldman KA, Enscore R, Lathrop S, et al. Outbreak of primary pneumonic tularemia on Martha's Vineyard. N Engl J Med 2001;345:1601--6.
- ↑ Smith S (2005-03-29). "City tells BU to bolster safety of its medical labs". Boston Globe. Retrieved 2007-05-09.
- ↑ Dvorak P (2005-10-2). "Health Officials Vigilant for Illness After Sensors Detect Bacteria on Mall: Agent Found as Protests Drew Thousands of Visitors". Washington Post. p. C13. Retrieved 2007-05-08.
A week after six bioterrorism sensors detected the presence of a dangerous bacterium on the Mall, health officials said there are no reports that any of the thousands of people in the nation's capital Sept. 24 have tularemia, the illness that results from exposure to the bacteria.
Check date values in:|date=(help)
- "Tularemia, NIAID Fact Sheet, April 2005". Retrieved 2007-01-07.
Template:Bacterial diseases cs:Tularémie de:Tularämie hr:Tularemija it:Tularemia no:Tularemi sr:Туларемија fi:Tularemia sv:Harpest