|
|
| Line 1: |
Line 1: |
| | __NOTOC__ |
| | |
| | '''For patient information click [[{{PAGENAME}} (patient information)|here]]''' |
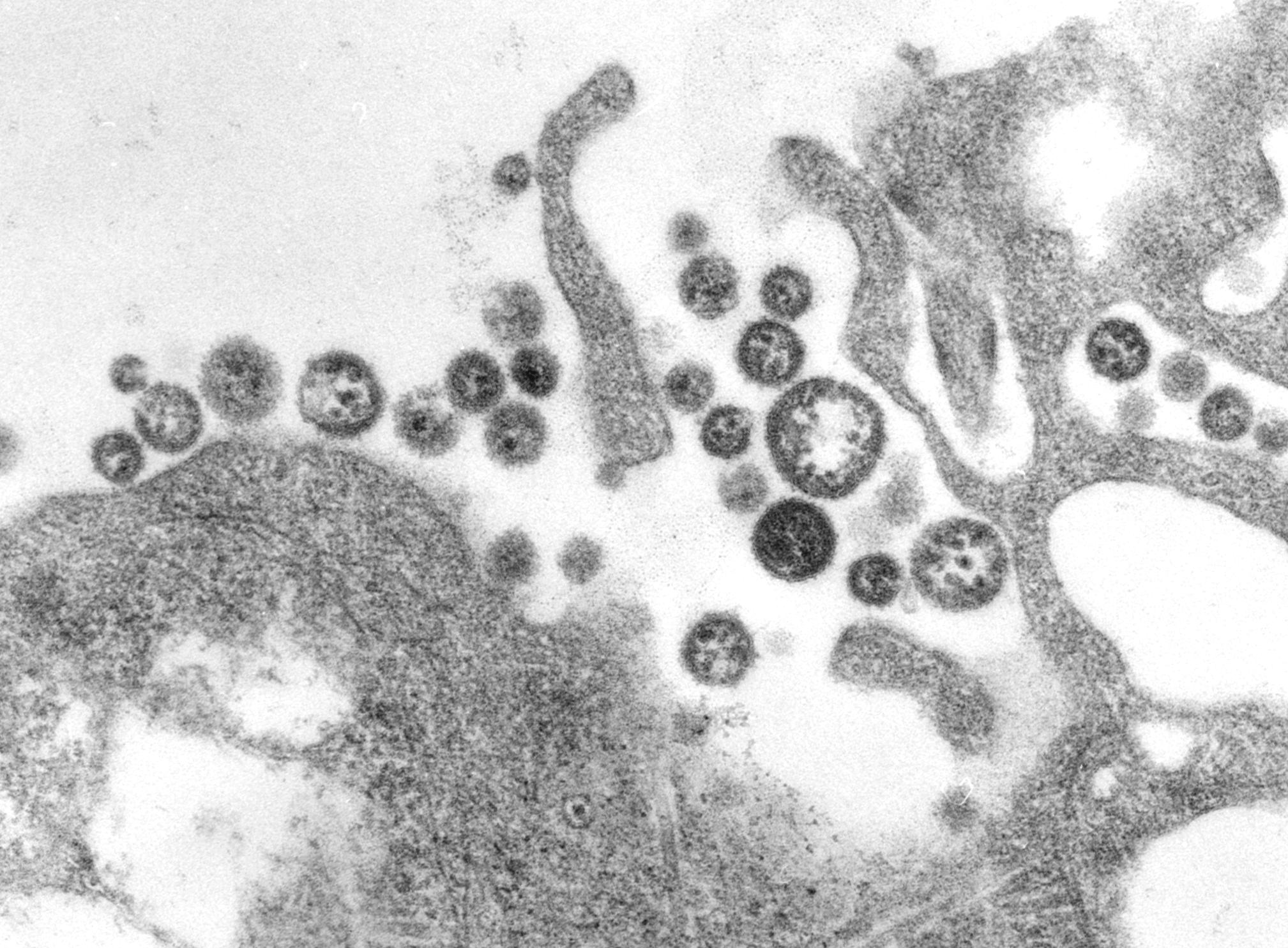
| {{Taxobox | color = violet | | {{Taxobox | color = violet |
| | name = ''Lassa virus'' | | | name = ''Lassa virus'' |
| Line 24: |
Line 27: |
| }} | | }} |
|
| |
|
| {{CMG}} | | {{Lassa fever}} |
| {{SI}}
| |
| | |
| | |
| '''Lassa fever''' is an acute [[virus|viral]] [[hemorrhagic fever]] first described in 1969 in the town of Lassa, Nigeria, located in the Yedseram river valley.<ref>{{cite journal |author=Frame JD, Baldwin JM, Gocke DJ, Troup JM |title=Lassa fever, a new virus disease of man from West Africa. I. Clinical description and pathological findings |journal=Am. J. Trop. Med. Hyg. |volume=19 |issue=4 |pages=670-6 |year=1970 |pmid=4246571 |url=http://www.ajtmh.org/cgi/content/abstract/19/4/670}}</ref> Clinical cases of the disease had been known for over a decade earlier but not connected with this viral pathogen. The infection is [[endemic (epidemiology)|endemic]] in West African countries, and causes 300-500,000 cases annually with ~5,000 deaths.<ref name=Ogbu_2007>{{cite journal |author=Ogbu O, Ajuluchukwu E, Uneke CJ |title=Lassa fever in West African sub-region: an overview |journal=Journal of vector borne diseases |volume=44 |issue=1 |pages=1-11 |year=2007 |pmid=17378212 |doi=}}</ref> Outbreaks of the disease have been observed in Nigeria, Liberia, Sierra Leone, Guinea, and the, Central African Republic, but it is believed that human infections also exist in Democratic Republic of the Congo, Mali, and Senegal.
| |
|
| |
|
| Lassa fever is also the most common hemorrhagic fever that is exported beyond its endemic area to countries like the United States, the United Kingdom of Great Britain and Northern Ireland, the Netherlands, Japan, and Israel.
| | {{CMG}}; '''Associate Editor-In-Chief:''' {{CZ}} |
|
| |
|
| ==Cause== | | ==[[Lassa fever overview|Overview]]== |
| Lassa fever is caused by the ''Lassa virus'', a member of the [[Arenaviridae]] family; it is an enveloped, single-stranded, bisegmented [[RNA]] virus.<ref name=Ogbu_2007 /> | |
|
| |
|
| Replication for Lassa virus is very rapid, while also demonstrating temporal control in replication. There are two [[genome]] segments. The first step involved is making [[messenger RNA|mRNA]] copies of the - sense genome. This ensures that there is adequate proteins, which are required for replication. The N and L proteins are made from the mRNA produced. The - sense genome then makes [[viral circular RNA|vcRNA]] copies of itself which are + sense. The vcRNA is a template for producing - sense progeny but mRNA is also synthesized from it. The mRNA synthesized from vcRNA translate the G (spike) proteins and Z proteins. Thus, with this temporal control, the spike proteins are produced last, making the infection further undetected by the host immune system.
| | ==[[Lassa fever historical perspective|Historical Perspective]]== |
|
| |
|
| Lassa virus will infect just about every tissue in the human body. It starts with the [[mucosa]], intestine, lungs and urinary systems, and then progresses to the vascular system.
| | ==[[Lassa fever pathophysiology|Pathophysiology]]== |
|
| |
|
| ==Pathophysiology== | | ==[[Lassa fever causes|Causes]]== |
| Infection in humans typically occurs via exposure to animal excrement through the [[respiratory tract|respiratory]] or [[gastrointestinal tract|gastrointestinal]] tracts. Inhalation of tiny particles of infective material (aerosol) is believed to be the most significant means of exposure. It is possible to acquire the infection through broken [[skin]] or [[mucous membrane]]s that are directly exposed to infective material. Transmission from person to person has also been established, presenting a disease risk for healthcare workers. Frequency of transmission via sexual contact has not been established.
| |
|
| |
|
| In 80% of cases the disease is inapparent, but in the remaining 20% it takes a complicated course. It is estimated that the virus is responsible for about 5,000 deaths annually. The fever accounts for up to ⅓ of deaths in hospitals within the affected regions and 10 to 16% of total cases.
| | ==[[Lassa fever differential diagnosis|Differentiating Lassa fever from other Diseases]]== |
|
| |
|
| After an [[incubation period]] of six to twenty-one days, an acute illness with multiorgan involvement develops. Non-specific symptoms include [[fever]], facial swelling, and muscle fatigue, as well as [[conjunctivitis]] and mucosal bleeding. The other symptoms arising from the affected organs are:
| | ==[[Lassa fever epidemiology and demographics|Epidemiology and Demographics]]== |
| * [[Gastrointestinal tract]]
| |
| ** [[nausea]]
| |
| ** [[vomit]]ing (bloody)
| |
| ** [[diarrhea]] (bloody)
| |
| ** [[stomach ache]]
| |
| ** [[constipation]]
| |
| ** [[dysphagia]] (difficulty swallowing)
| |
| ** [[hepatitis]]
| |
|
| |
|
| * [[Cardiovascular system]]
| | ==[[Lassa fever risk factors|Risk Factors]]== |
| ** [[pericarditis]]
| |
| ** [[hypertension]]
| |
| ** [[hypotension]]
| |
| ** [[tachycardia]] (abnormally high heart rate)
| |
|
| |
|
| * [[Respiratory tract]]
| | ==[[Lassa fever natural history, complications and prognosis|Natural History, Complications and Prognosis]]== |
| ** [[cough]]
| |
| ** chest pain
| |
| ** [[dyspnoea]]
| |
| ** [[pharyngitis]]
| |
| ** [[pleuritis]]
| |
|
| |
|
| * [[Nervous system]]
| | ==Diagnosis== |
| ** [[encephalitis]]
| |
| ** [[meningitis]]
| |
| ** unilateral or bilateral hearing deficit
| |
| ** [[seizure]]s
| |
|
| |
|
| Clinically, Lassa fever infections are difficult to distinguish from other viral hemorrhagic fevers such as [[Ebola]] and [[Marburg virus|Marburg]], and from more common febrile illnesses such as [[malaria]].
| | [[Lassa fever history and symptoms|History and Symptoms]] | [[Lassa fever physical examination|Physical Examination]] | [[Lassa fever laboratory findings|Laboratory Findings]] | [[Lassa fever other diagnostic studies|Other Diagnostic Studies]] |
| | |
| The virus is excreted in urine for three to nine weeks and in semen for three months.
| |
|
| |
|
| ==Treatment== | | ==Treatment== |
| All persons suspected of Lassa fever infection should be admitted to isolation facilities and their body fluids and excreta properly disposed of.
| |
|
| |
| Early and aggressive treatment using [[Ribavirin]] was pioneered by [[Joseph B. McCormick|Joe McCormick]] in 1979. After extensive testing, it was determined that early administration is critical to success. Additionally, Ribavirin is almost twice as effective when given intravenously as when taken by mouth.<ref>{{cite journal |author=Fisher-Hoch SP, McCormick JB |title=Lassa fever vaccine |journal=Expert review of vaccines |volume=3 |issue=2 |pages=189-97 |year=2004 |pmid=15056044 |doi=10.1586/14760584.3.4.S189}}</ref> Ribavirin is a [[prodrug]] which appears to interfere with viral replication by inhibiting RNA-dependent [[DNA Replication|nucleic acid synthesis]], although the precise [[mechanism of action]] is disputed.<ref>{{cite journal |author=Crotty S, Cameron C, Andino R |title=Ribavirin's antiviral mechanism of action: lethal mutagenesis? |journal=J. Mol. Med. |volume=80 |issue=2 |pages=86-95 |year=2002 |pmid=11907645 |doi=10.1007/s00109-001-0308-0}}</ref> The drug is relatively inexpensive, but the cost of the drug is still very high for many of those in poverty-stricken West African states. Fluid replacement, blood transfusion and fighting hypotension are usually required. Intravenous [[interferon]] therapy has also been used.
| |
|
| |
| When Lassa fever infects pregnant women late in their third trimester, it is necessary to abort the pregnancy for the mother to have a good chance of survival.<ref>{{cite journal |author=Price ME, Fisher-Hoch SP, Craven RB, McCormick JB |title=A prospective study of maternal and fetal outcome in acute Lassa fever infection during pregnancy |journal=BMJ |volume=297 |issue=6648 |pages=584–7 |year=1988 |pmid=3139220 |url=http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=3139220}}</ref> This is due to the fact that the virus has an affinity for the placenta and other highly vascular tissues. The fetus has only a one in ten chance of survival no matter what course of action is taken; hence focus is always on saving the life of the mother. Following abortion, women should receive the same treatment as other Lassa fever patients.
| |
|
| |
| Siga Technologies is developing an [[antiviral drug]] that has been shown effective in treating experimentally infected guinea pigs. In a study conducted at the [[USAMRIID|U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID)]], treatment with ST-193 once a day for 14 days resulted in significant reduction in mortality (71% of the animals survived at the low dose), whereas all untreated animals and those treated with [[ribavirin]] died within 20 days of the infection.<ref>{{cite press release
| |
| | url = http://www.siga.com/press/051507.html
| |
| | title = SIGA Passes First Hurdle with Lassa Fever Antiviral ST-193}}</ref>
| |
|
| |
| ==Prognosis==
| |
| About 15%-20% of hospitalized Lassa fever patients will die from the illness. It is estimated that the overall mortality rate is 1%, however during [[epidemic]]s mortality can climb as high as 50%. Also the mortality rate is higher, greater than 80%, when it occurs in pregnant women during their third trimester; fetal death also occurs in nearly all those cases. Abortion decreases the risk of death to the mother.
| |
|
| |
| Thanks to treatment with [[Ribavirin]], fatality rates are continuing to decline. Work on a vaccine is continuing, with multiple approaches showing positive results in animal trials.
| |
|
| |
| ==Epidemiology==
| |
| Lassa virus is [[zoonosis|zoonotic]] (transmitted from animals), and that it spreads to man from [[rodent]]s, specifically multi-mammate rats (''[[Mastomys natalensis]]''). This is probably the most common rodent in equatorial Africa, ubiquitous in human households and eaten as a delicacy in some areas.{{Fact|date=August 2007}} In these rats infection is in a persistent [[asymptomatic]] state. The virus is shed in their excreta (urine and feces), which can be aerosolized. In fatal cases, Lassa fever is characterized by impaired or delayed cellular immunity leading to [[fulminant]] [[viremia]].
| |
|
| |
| The dissemination of the infection can be assessed by prevalence of antibodies to the virus in populations of:
| |
| *Sierra Leone 8–52%
| |
| *Guinea 4–55%
| |
| *Nigeria approx. 21%
| |
|
| |
| Like other [[Viral hemorrhagic fever|hemorrhagic fevers]], Lassa fever can be transmitted directly from one human to another. It can be contracted by an airborne route or with direct contact with infected human blood, urine, or semen. Transmission through [[breast milk]] has also been observed.
| |
|
| |
| ==Lab tests==
| |
| There is a range of laboratory investigations that are performed to diagnose the disease and assess its course and complications. [[ELISA test]] for antigen and [[IgM]] antibodies gives 88% sensitivity and 90% specificity for the presence of the infection. Other laboratory findings in Lassa fever include [[lymphopenia]] (low white blood cell count), [[thrombocytopenia]] (low platelets), and elevated [[aspartate aminotransferase]] (AST) levels in the blood.
| |
|
| |
| ==Prevention==
| |
| Control of the ''Mastomys'' rodent population is impractical, so measures are limited to keeping rodents out of homes and food supplies, as well as maintaining effective personal hygiene. Gloves, masks, laboratory coats, and goggles are advised while in contact with an infected person.
| |
|
| |
|
| No [[vaccine]] against Lassa fever is currently available, though development is underway. The Mozambique virus closely resembles Lassa fever, while lacking its deadly effects. This virus is being considered for possible use as a vaccine.
| | [[Lassa fever medical therapy|Medical Therapy]] | [[Lassa fever primary prevention|Primary Prevention]] | [[Lassa fever secondary prevention|Secondary Prevention]] | [[Lassa fever cost-effectiveness of therapy|Cost-Effectiveness of Therapy]] | [[Lassa fever future or investigational therapies|Future or Investigational Therapies]] |
|
| |
|
| Researchers at the [[USAMRIID]] facility, where military biologists study infectious diseases, have a promising vaccine candidate.<ref>[[Richard Preston|Preston, Richard]]. 2002 ''The Demon In The Freezer''. Random House, Inc.</ref> They have developed a [[Virus#Replication|replication]]-competent vaccine against Lassa virus based on recombinant vesicular [[stomatitis]] [[Vesicular stomatitis virus|virus]] vectors expressing the Lassa virus glycoprotein. After a single [[Intramuscular|intramuscular injection]], test primates have survived lethal challenge, while showing no clinical symptoms.<ref>{{cite journal |author=Geisbert TW, Jones S, Fritz EA, ''et al'' |title=Development of a new vaccine for the prevention of Lassa fever |journal=PLoS Med. |volume=2 |issue=6 |pages=e183 |year=2005 |pmid=15971954 |doi=10.1371/journal.pmed.0020183}}</ref>
| | ==Case Studies== |
| | [[Lassa fever case study one|Case #1]] |
|
| |
|
| ==References== | | ==References== |
| Line 125: |
Line 70: |
| * [http://www.cdc.gov/ncidod/diseases/virlfvr/virlfvr.htm CDC info - viral fevers] | | * [http://www.cdc.gov/ncidod/diseases/virlfvr/virlfvr.htm CDC info - viral fevers] |
| * [http://www.hpa.org.uk/infections/topics_az/VHF/menu.htm Health Protection Agency - viral haemorrhagic fevers] | | * [http://www.hpa.org.uk/infections/topics_az/VHF/menu.htm Health Protection Agency - viral haemorrhagic fevers] |
| * [http://www.merlin.org.uk/Main.aspxPageID=178 Merlin]
| |
|
| |
|
| {{Viral diseases}} | | {{Viral diseases}} |