Acute myeloid leukemia pathophysiology: Difference between revisions
| Line 41: | Line 41: | ||
|-valign="top" | |-valign="top" | ||
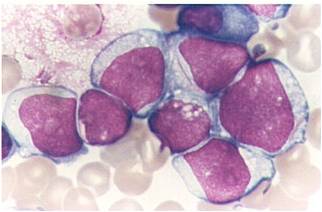
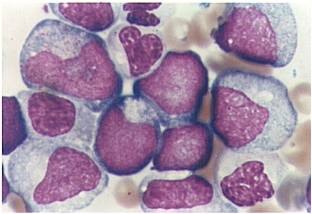
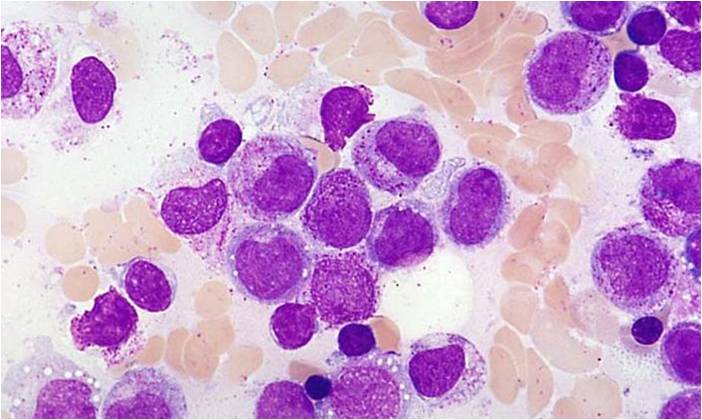
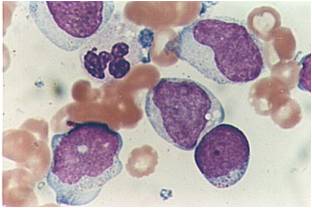
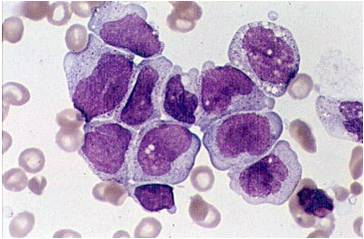
| [[Image:AML-M2 0002.jpg|thumb|AML-M2 - presence of granules can be noted]] | | [[Image:AML-M2 0002.jpg|thumb|AML-M2 - presence of granules can be noted]] | ||
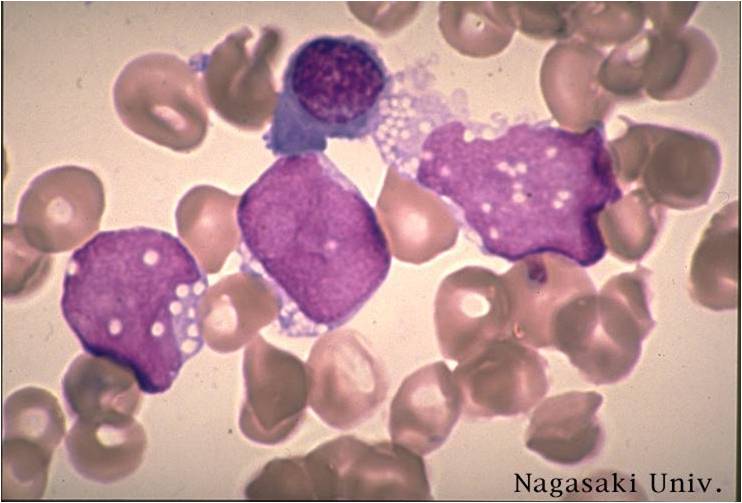
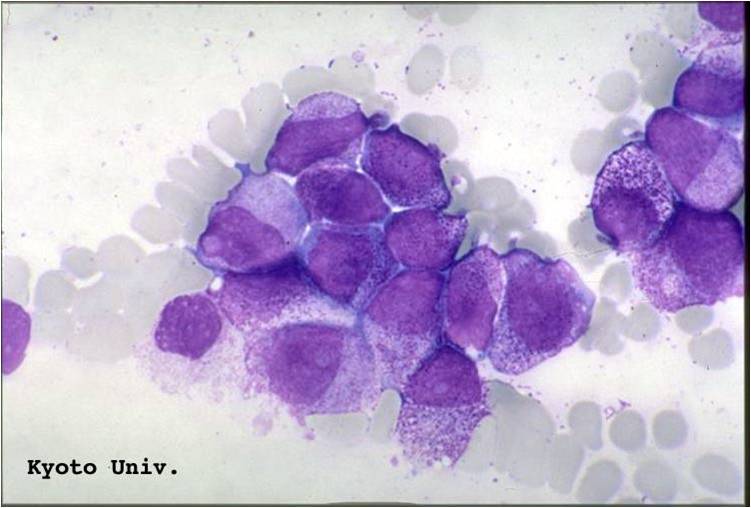
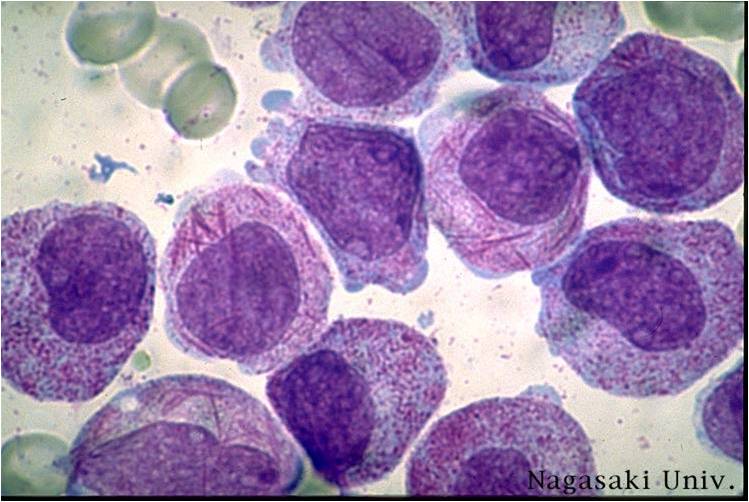
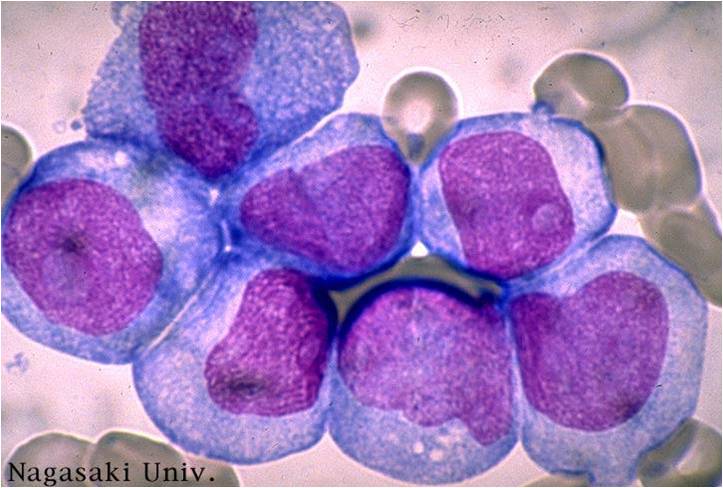
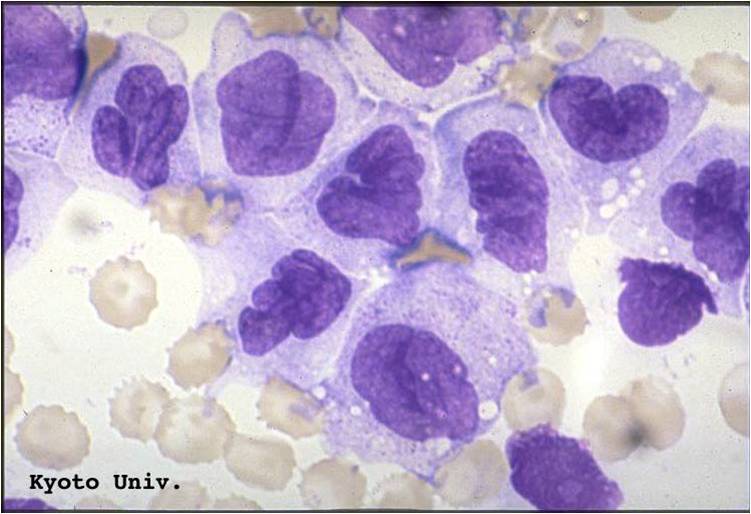
| [[Image:AML-M2 0001.jpg|thumb|AML-M2]] | | [[Image:AML-M2 0001.jpg|thumb|AML-M2 - large myeloblasts with prominent nucleoli]] | ||
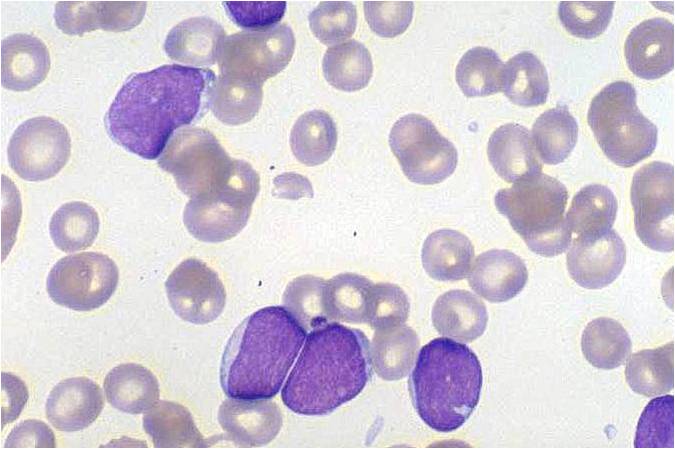
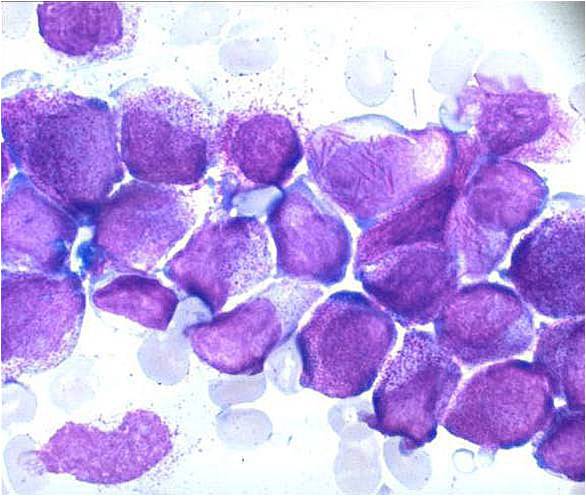
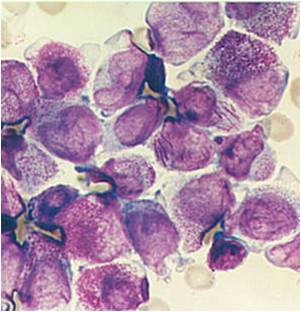
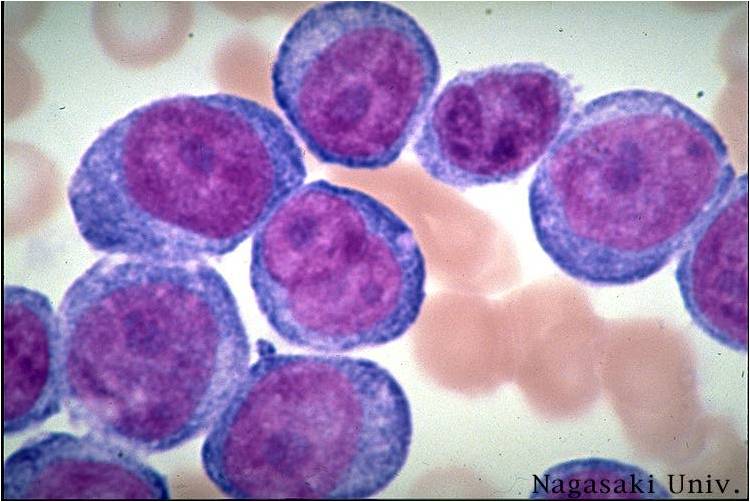
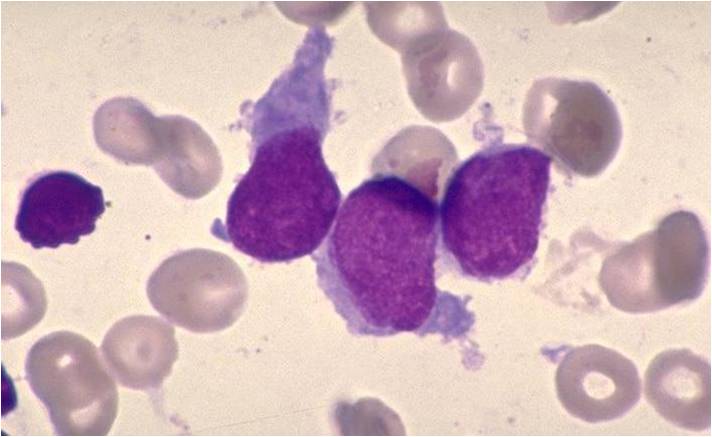
| [[Image:AML-M2 0004.jpg|thumb|AML-M2]] | | [[Image:AML-M2 0004.jpg|thumb|AML-M2]] | ||
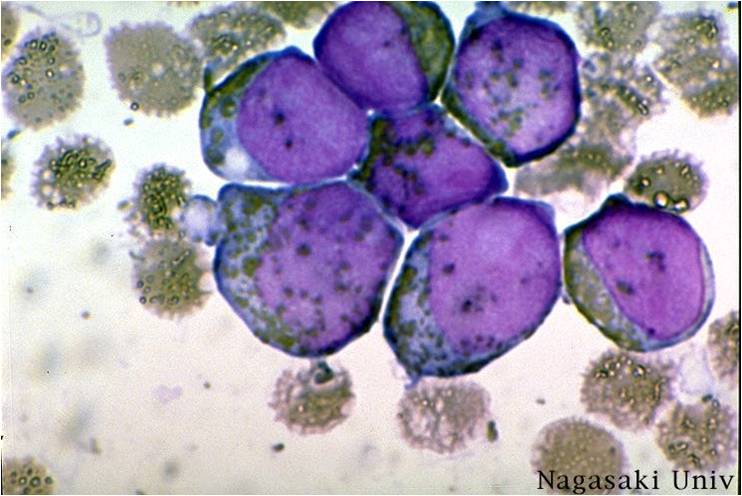
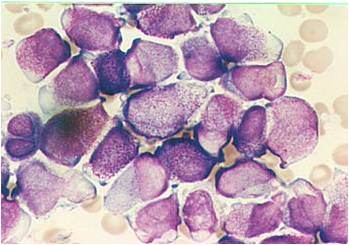
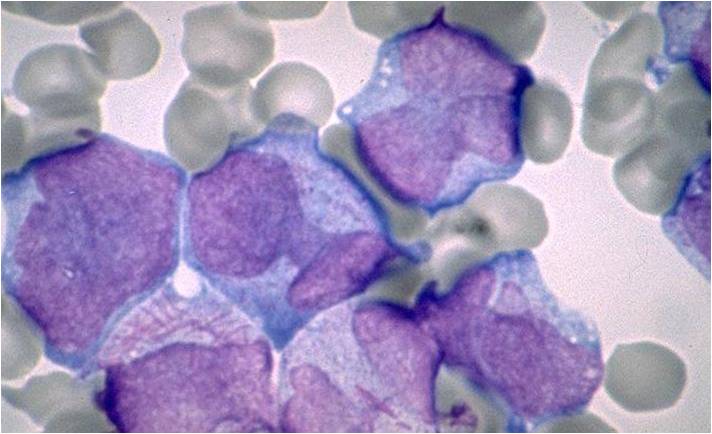
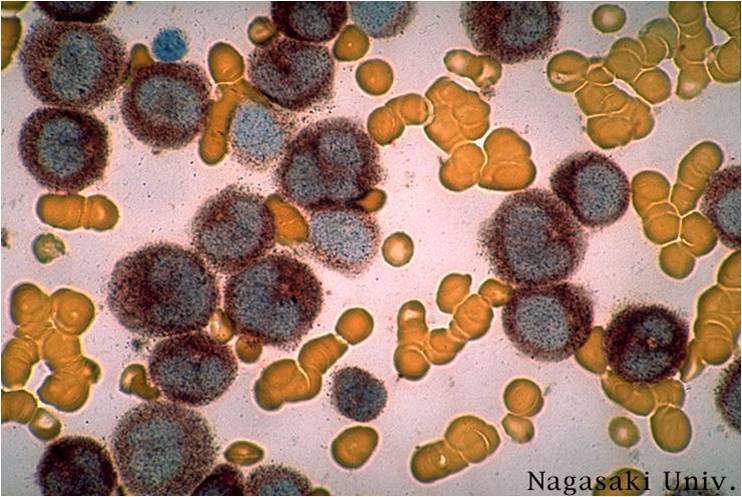
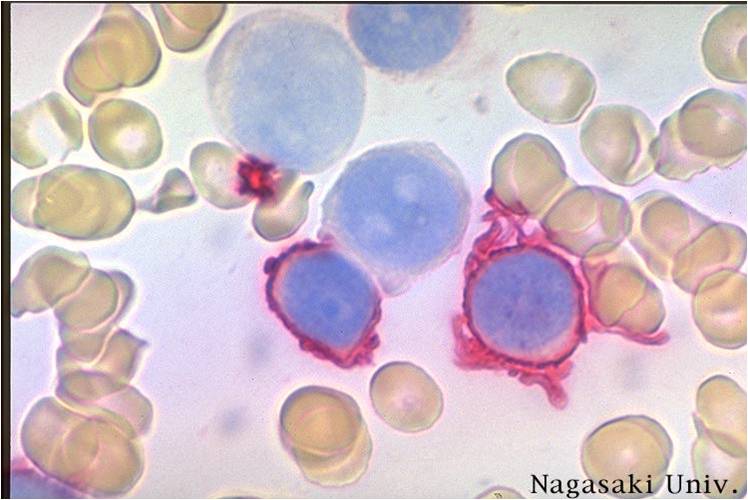
| [[Image:AML-M2 0003.jpg|thumb|AML-M2]] | | [[Image:AML-M2 0003.jpg|thumb|AML-M2]] | ||
Revision as of 15:08, 8 August 2012
|
Acute myeloid leukemia Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Acute myeloid leukemia pathophysiology On the Web |
|
American Roentgen Ray Society Images of Acute myeloid leukemia pathophysiology |
|
Risk calculators and risk factors for Acute myeloid leukemia pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Raviteja Guddeti, M.B.B.S. [2]
Overview
Pathophysiology
The malignant cell in AML is the myeloblast. In normal hematopoiesis, the myeloblast is an immature precursor of myeloid white blood cells; a normal myeloblast will gradually mature into a mature white blood cell. However, in AML, a single myeloblast accumulates genetic changes which "freeze" the cell in its immature state and prevent differentiation.[1] Such a mutation alone does not cause leukemia; however, when such a "differentiation arrest" is combined with other mutations which disrupt genes controlling proliferation, the result is the uncontrolled growth of an immature clone of cells, leading to the clinical entity of AML.[2]
Much of the diversity and heterogeneity of AML stems from the fact that leukemic transformation can occur at a number of different steps along the differentiation pathway.[3] Modern classification schemes for AML recognize that the characteristics and behavior of the leukemic cell (and the leukemia) may depend on the stage at which differentiation was halted.
Specific cytogenetic abnormalities can be found in many patients with AML; the types of chromosomal abnormalities often have prognostic significance.[4] The chromosomal translocations encode abnormal fusion proteins, usually transcription factors whose altered properties may cause the "differentiation arrest."[5] For example, in acute promyelocytic leukemia, the t(15;17) translocation produces a PML-RARα fusion protein which binds to the retinoic acid receptor element in the promoters of several myeloid-specific genes and inhibits myeloid differentiation.[6]
The clinical signs and symptoms of AML result from the fact that, as the leukemic clone of cells grows, it tends to displace or interfere with the development of normal blood cells in the bone marrow.[7] This leads to neutropenia, anemia, and thrombocytopenia. The symptoms of AML are in turn often due to the low numbers of these normal blood elements. In rare cases, patients can develop a chloroma, or solid tumor of leukemic cells outside the bone marrow, which can cause various symptoms depending on its location.
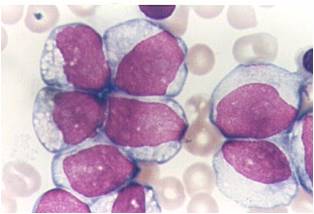
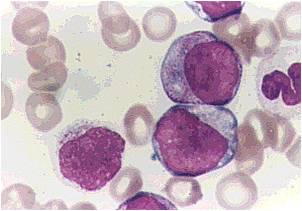
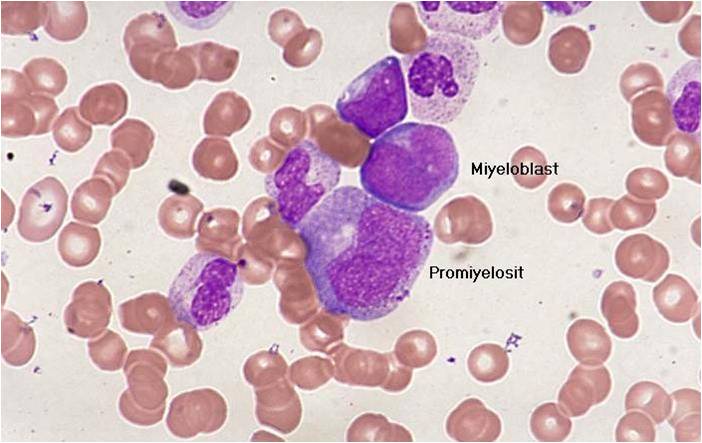
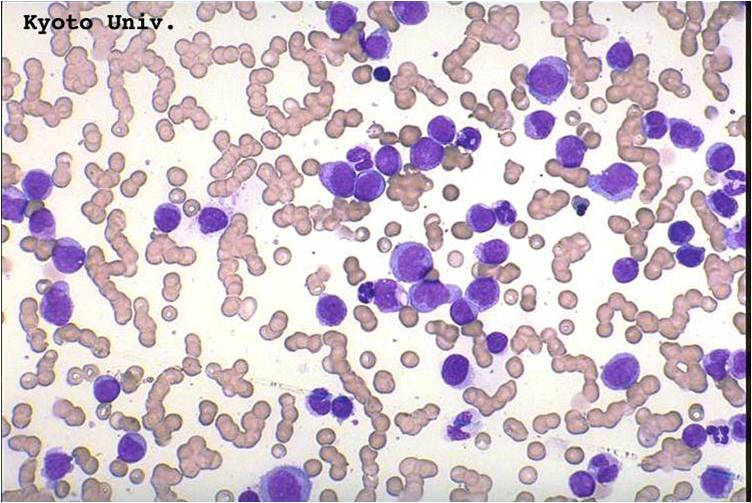
Microscopic Pathology
(Images shown below are courtesy of Melih Aktan MD., Istanbul Medical Faculty - Turkey, and Kyoto University - Japan)
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
References
- ↑ Fialkow PJ: Clonal origin of human tumors. Biochim Biophys Acta 1976;458:283–321. PMID 1067873
- ↑ Fialkow PJ, Janssen JW, Bartram CR: Clonal remissions in acute nonlymphocytic leukemia: Evidence for a multistep pathogenesis of the malignancy. Blood 1991;77:1415–1517. PMID 2009365
- ↑ Bonnet D, Dick JE: Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 1997;3:730–737. PMID 9212098
- ↑ Abeloff, Martin et al. (2004), pp. 2831–32.
- ↑ Greer, John P., et al. Wintrobe's Clinical Hematology, 11th ed. Philadelphia: Lippincott, Williams, and Wilkins, 2004. p. 2045–2062
- ↑ Melnick A, Licht JD. Deconstructing a disease: RARα its fusion partners, and their roles in the pathogenesis of acute promyelocytic leukemia. Blood 1999;93:3167–3215. PMID 10233871
- ↑ Abeloff, Martin et al. (2004), p. 2828.