Pulmonary embolism CT: Difference between revisions
No edit summary |
|||
| Line 77: | Line 77: | ||
====[[European society of cardiology#Classes of Recommendations|Class IIa B]]==== | ====[[European society of cardiology#Classes of Recommendations|Class IIa B]]==== | ||
'''1.''' In patients with a negative CT, further tests should be considered in selected patients to exclude pulmonary embolism. ''([[European society of cardiology#Level of Evidence|Level of Evidence: B]])''}} | '''1.''' In patients with a negative CT, further tests should be considered in selected patients to exclude pulmonary embolism. ''([[European society of cardiology#Level of Evidence|Level of Evidence: B]])''}} | ||
==Pulmonary septic emboli== | |||
Septic emboli are seen most commonly in: | |||
* Patients with infective endocarditis | |||
* Patients with infected venous catheters or pacemaker leads | |||
* Patients with periodontal disease | |||
=== CT === | |||
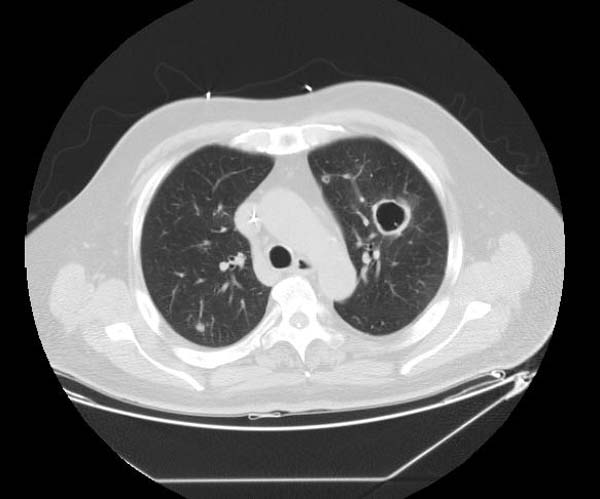
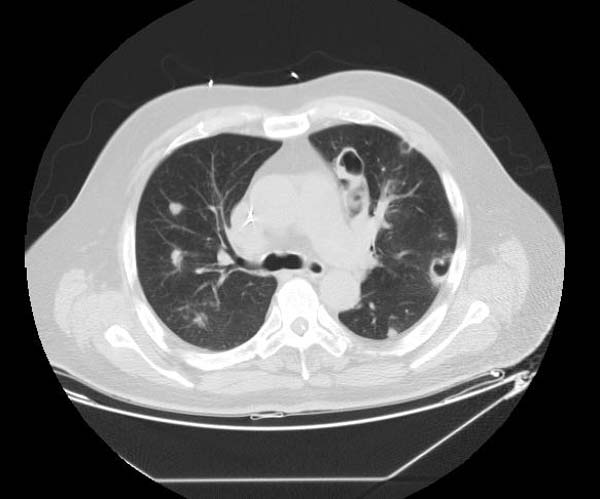
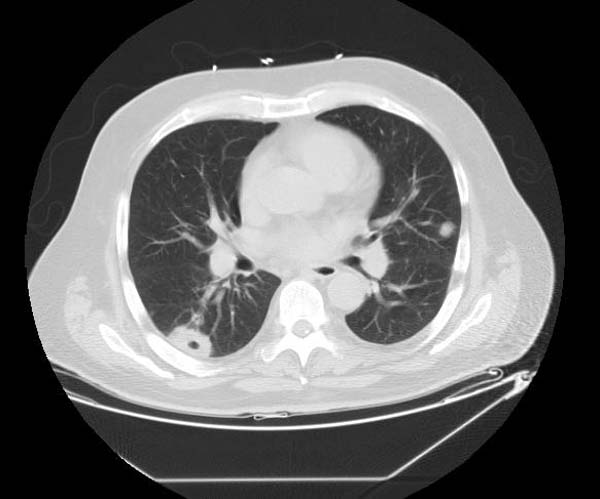
* The CT appearance of septic emboli includes '''nodules''' and '''wedge-shaped subpleural opacities with or without cavitation''' and the feeding vessel sign]. | |||
* The feeding vessel sign consists of a distinct vessel leading directly into the center of a nodule. This sign has been considered highly suggestive of septic embolism, the prevalence varying from 67-100% in various series (note: the feeding vessel sign also occurs in pulmonary metastasis). | |||
<gallery> | |||
Image: | |||
Septic-emboli-001.jpg|Pulmonary septic emboli | |||
Image: | |||
Septic-emboli-002.jpg|Pulmonary septic emboli | |||
Image: | |||
Septic-emboli-003.jpg|Pulmonary septic emboli | |||
</gallery> | |||
==Guideline Resources== | ==Guideline Resources== | ||
Revision as of 17:14, 2 August 2012
|
Pulmonary Embolism Microchapters |
|
Diagnosis |
|---|
|
Pulmonary Embolism Assessment of Probability of Subsequent VTE and Risk Scores |
|
Treatment |
|
Follow-Up |
|
Special Scenario |
|
Trials |
|
Case Studies |
|
Pulmonary embolism CT On the Web |
Editor(s)-In-Chief: The APEX Trial Investigators, C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]
Overview
Contrast pulmonary angiography is the gold standard when diagnosing a PE. The disadvantages of using pulmonary angiography are its invasiveness, high costs, limited availability, and the need of an expert radiologist. This chapter deals with the advantages of multidetector CT over CTPA.
Single-Detector CT
Recent improvements in CT technology have reduced the value of CT angiography for the initial workup of PE patients. Studies surveying single detector spiral CT use in cases of suspected pulmonary embolism, show wide variations in both sensitivity (53-100%) and specificity (73-100%) for detecting a PE.[1][2]
Two large multicentric clinical studies for single-detector CT, including more than 1000 patients, reported a sensitivity of 70% and a specificity of 90% for the diagnosis of a PE.[3][4] Due to motion artifacts and insufficient opacification, the rate of technical inadequacy of single detector CT in this study was 5-8%.
Two large studies have shown that a combination of a negative single detector CT and an the absence of proximal lower limb DVT on lower limb venous ultrasonoagraphy in non-high clinical probability patients were associated with a 3-month thromboembolic risk of 1%.[5][6]
Multi-Detector CT
Since its introduction, CT angiography has been the method of choice for imaging the pulmonary vasculature for suspected PE patients in routine clinical practice. Although CT angiography remains the gold standard in diagnosing a PE, MDCT and SDCT are often the initial modes of evaluating patients with a suspected PE. In comparison to angiography, a CT is less invasive, takes less time, is easier to perform, and exposes the patient to lower amounts of radiation.
Advantage of MDCT over SDCT
- High spatial resolution.
- High temporal resolution.
- Better quality of arterial opacification.
- Adequate visualization of pulmonary arteries up to at least the segmental level.
Supportive Trial Date
A study enrolling 94 patients, done in 2004, showed the sensitivity and specificity of multi-detector CT to be above 90% in the diagnosis of pulmonary embolism.[7]
The PIOPED II study which enrolled 824 patients, published their results in 2006 showing a sensitivity and specificity of multi-detector CT to be 83% and 96% respectively in the diagnosis of PE.[8] Though PIOPED II study also highlighted the influence of clinical probability on the predictive value of MDCT.
Another study with enrollment of 1819 patients, compared two diagnostic strategies based on D-dimer and MDCT, one with and the other without lower limb compression ultrasonography (CUS). The study reported that the 3-month thromboembolic risk was 0.3% (95% CI 0.1-1.1) in the D-dimer-Ultrasonography-CT (DD-US-CT) group and 0.3% (0.1-1.2) in the DD-CT group (difference 0.0% [-0.9 to 0.8]). In the DD-US-CT group, ultrasonography showed a deep-venous thrombosis in 53 (9% [7-12]) of 574 patients, and thus MDCT was not undertaken.[9]
Role of MDCT in diagnosing PE[10]
- In patients with a non-high clinical probability of PE, a negative MDCT is adequate criteria for excluding PE.
- In patients with high a clinical probability of PE, and a negative CT, there is still some disagreement as to whether there should be further investigation by compression ultrasonography and/or ventilation-perfusion (V/Q) scan or pulmonary angiography.[10]
- In patients with non-low clinical probability of PE:
- A MDCT showing PE at segmental or more proximal level is an adequate proof of PE in those patients.
- According to PIOPED II, the PPV of MDCT was found to be low (58%)[8] so further testing should be considered in the case of a negative MDCT.
Role of MDCT in assessment of right ventricular dysfunction
Right ventricular dysfunction is an independent predictor of clinical deterioration and death in pulmonary embolism patients, therefore it can be used for risk stratification for adverse outcomes. Thus MDCT has the potential to provide both diagnostic and prognostic stratification in acute pulmonary embolism patients.
- In a study, a right-to-left ventricular dimensional ratio of less than 0.9 on MDCT was found to have a 100% NPV for death due to PE.[11]
- This could also be used to identify those patients at low risk of death, who are candidates for early discharge or home treatment.
Isolated subsegmental PE: Role of MDCT in deciding treatment
Presence of a single subsegmental clot on MDCT is termed as isolated subsegmental PE. 1-5 % of patients with suspected PE undergoing MDCT have found to have isolated subsegmental PE.[12][13][14]
- Positive predictable value of such finding is low.
- Outcome studies have showed that patient left untreated by anticoagulants may have uneventful course.
- Compression ultrasonography is advised to rule out DVT, to assist treatment in patients with isolated subsegmental PE.
- In patients with isolated subsegmental PE, but without DVT, no recommendation is made due to lack of evidence.[10]
ESC Guideline Recommendations [10]
Suspected High-risk PE Patients
| “ |
Class I1. In high-risk pulmonary embolism, as indicated by the presence of shock or hypotension, emergency CT or bedside echocardiography (depending on availability and clinical circumstances) is recommended for diagnostic purposes. (Level of Evidence: C) |
” |
Suspected Non High-risk PE Patients
| “ |
Low clinical probabilityClass I1. Normal D-dimer level using either a highly or moderately sensitive assay excludes pulmonary embolism. (Level of Evidence: A) Intermediate clinical probabilityClass I1. Negative multi-detector CT (MDCT) safely excludes pulmonary embolism. (Level of Evidence: A) 2. Negative single-detector CT (SDCT) only excludes pulmonary embolism when combined with negative proximal compression ultrasonography. (Level of Evidence: A) 3. SDCT or MDCT showing a segmental or more proximal thrombus confirms pulmonary embolism. (Level of Evidence: A) Class IIa1. Further testing should be considered to confirm pulmonary embolsim if SDCT or MDCT shows only subsegmental clots. (Level of Evidence: B) High clinical probabilityClass I1. SDCT or MDCT showing a segmental or more proximal thrombus confirms pulmonary embolism. (Level of Evidence: A) Class IIa B1. In patients with a negative CT, further tests should be considered in selected patients to exclude pulmonary embolism. (Level of Evidence: B) |
” |
Pulmonary septic emboli
Septic emboli are seen most commonly in:
- Patients with infective endocarditis
- Patients with infected venous catheters or pacemaker leads
- Patients with periodontal disease
CT
- The CT appearance of septic emboli includes nodules and wedge-shaped subpleural opacities with or without cavitation and the feeding vessel sign].
- The feeding vessel sign consists of a distinct vessel leading directly into the center of a nodule. This sign has been considered highly suggestive of septic embolism, the prevalence varying from 67-100% in various series (note: the feeding vessel sign also occurs in pulmonary metastasis).
-
Pulmonary septic emboli
-
Pulmonary septic emboli
-
Pulmonary septic emboli
Guideline Resources
Guidelines on the diagnosis and management of acute pulmonary embolism. The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology[10]
References
- ↑ Mullins MD, Becker DM, Hagspiel KD, Philbrick JT (2000). "The role of spiral volumetric computed tomography in the diagnosis of pulmonary embolism". Arch. Intern. Med. 160 (3): 293–8. PMID 10668830. Retrieved 2012-04-30. Unknown parameter
|month=ignored (help) - ↑ Rathbun SW, Raskob GE, Whitsett TL (2000). "Sensitivity and specificity of helical computed tomography in the diagnosis of pulmonary embolism: a systematic review". Ann. Intern. Med. 132 (3): 227–32. PMID 10651604. Retrieved 2012-04-30. Unknown parameter
|month=ignored (help) - ↑ Perrier A, Howarth N, Didier D, Loubeyre P, Unger PF, de Moerloose P, Slosman D, Junod A, Bounameaux H (2001). "Performance of helical computed tomography in unselected outpatients with suspected pulmonary embolism". Ann. Intern. Med. 135 (2): 88–97. PMID 11453707. Retrieved 2012-04-30. Unknown parameter
|month=ignored (help) - ↑ Van Strijen MJ, De Monye W, Kieft GJ, Pattynama PM, Prins MH, Huisman MV (2005). "Accuracy of single-detector spiral CT in the diagnosis of pulmonary embolism: a prospective multicenter cohort study of consecutive patients with abnormal perfusion scintigraphy". J. Thromb. Haemost. 3 (1): 17–25. doi:10.1111/j.1538-7836.2004.01064.x. PMID 15634261. Retrieved 2012-04-30. Unknown parameter
|month=ignored (help) - ↑ Musset D, Parent F, Meyer G, Maître S, Girard P, Leroyer C, Revel MP, Carette MF, Laurent M, Charbonnier B, Laurent F, Mal H, Nonent M, Lancar R, Grenier P, Simonneau G (2002). "Diagnostic strategy for patients with suspected pulmonary embolism: a prospective multicentre outcome study". Lancet. 360 (9349): 1914–20. doi:10.1016/S0140-6736(02)11914-3. PMID 12493257. Retrieved 2012-04-30. Unknown parameter
|month=ignored (help) - ↑ Perrier A, Roy PM, Aujesky D, Chagnon I, Howarth N, Gourdier AL, Leftheriotis G, Barghouth G, Cornuz J, Hayoz D, Bounameaux H (2004). "Diagnosing pulmonary embolism in outpatients with clinical assessment, D-dimer measurement, venous ultrasound, and helical computed tomography: a multicenter management study". Am. J. Med. 116 (5): 291–9. doi:10.1016/j.amjmed.2003.09.041. PMID 14984813. Retrieved 2012-04-30. Unknown parameter
|month=ignored (help) - ↑ Winer-Muram HT, Rydberg J, Johnson MS, Tarver RD, Williams MD, Shah H, Namyslowski J, Conces D, Jennings SG, Ying J, Trerotola SO, Kopecky KK (2004). "Suspected acute pulmonary embolism: evaluation with multi-detector row CT versus digital subtraction pulmonary arteriography". Radiology. 233 (3): 806–15. doi:10.1148/radiol.2333031744. PMID 15564410. Retrieved 2012-05-01. Unknown parameter
|month=ignored (help) - ↑ 8.0 8.1 Stein PD, Fowler SE, Goodman LR, Gottschalk A, Hales CA, Hull RD, Leeper KV, Popovich J, Quinn DA, Sos TA, Sostman HD, Tapson VF, Wakefield TW, Weg JG, Woodard PK (2006). "Multidetector computed tomography for acute pulmonary embolism". N. Engl. J. Med. 354 (22): 2317–27. doi:10.1056/NEJMoa052367. PMID 16738268. Retrieved 2012-05-01. Unknown parameter
|month=ignored (help) - ↑ Righini M, Le Gal G, Aujesky D, Roy PM, Sanchez O, Verschuren F, Rutschmann O, Nonent M, Cornuz J, Thys F, Le Manach CP, Revel MP, Poletti PA, Meyer G, Mottier D, Perneger T, Bounameaux H, Perrier A (2008). "Diagnosis of pulmonary embolism by multidetector CT alone or combined with venous ultrasonography of the leg: a randomised non-inferiority trial". Lancet. 371 (9621): 1343–52. doi:10.1016/S0140-6736(08)60594-2. PMID 18424324. Retrieved 2012-05-01. Unknown parameter
|month=ignored (help) - ↑ 10.0 10.1 10.2 10.3 10.4 Torbicki A, Perrier A, Konstantinides S, Agnelli G, Galiè N, Pruszczyk P, Bengel F, Brady AJ, Ferreira D, Janssens U, Klepetko W, Mayer E, Remy-Jardin M, Bassand JP (2008). "Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC)". Eur. Heart J. 29 (18): 2276–315. doi:10.1093/eurheartj/ehn310. PMID 18757870. Retrieved 2012-05-01. Unknown parameter
|month=ignored (help) - ↑ Becattini C, Agnelli G, Vedovati MC, Pruszczyk P, Casazza F, Grifoni S, Salvi A, Bianchi M, Douma R, Konstantinides S, Lankeit M, Duranti M (2011). "Multidetector computed tomography for acute pulmonary embolism: diagnosis and risk stratification in a single test". Eur. Heart J. 32 (13): 1657–63. doi:10.1093/eurheartj/ehr108. PMID 21504936. Retrieved 2012-05-01. Unknown parameter
|month=ignored (help) - ↑ Perrier A, Roy PM, Sanchez O, Le Gal G, Meyer G, Gourdier AL; et al. (2005). "Multidetector-row computed tomography in suspected pulmonary embolism". N Engl J Med. 352 (17): 1760–8. doi:10.1056/NEJMoa042905. PMID 15858185. Review in: J Fam Pract. 2005 Aug;54(8):653, 657
- ↑ Brunot S, Corneloup O, Latrabe V, Montaudon M, Laurent F (2005). "Reproducibility of multi-detector spiral computed tomography in detection of sub-segmental acute pulmonary embolism". Eur Radiol. 15 (10): 2057–63. doi:10.1007/s00330-005-2844-4. PMID 16021452.
- ↑ Eyer BA, Goodman LR, Washington L (2005). "Clinicians' response to radiologists' reports of isolated subsegmental pulmonary embolism or inconclusive interpretation of pulmonary embolism using MDCT". AJR Am J Roentgenol. 184 (2): 623–8. PMID 15671388.