Axicabtagene ciloleucel: Difference between revisions
No edit summary |
No edit summary |
||
| Line 309: | Line 309: | ||
|PK=(Description) | |PK=(Description) | ||
|nonClinToxic=(Description) | |nonClinToxic=(Description) | ||
|clinicalStudies====== | |clinicalStudies======Relapsed or Refractory Large B-Cell Lymphoma===== | ||
( | *A single-arm, open-label, multicenter trial evaluated the efficacy of a single infusion of YESCARTA in adult patients with relapsed or refractory aggressive B-cell non-Hodgkin lymphoma. Eligible patients had refractory disease to the most recent therapy or relapse within 1 year after autologous hematopoietic stem cell transplantation (HSCT). The study excluded patients with prior allogeneic HSCT, any history of central nervous system lymphoma, ECOG performance status of 2 or greater, absolute lymphocyte count less than 100/µL, creatinine clearance less than 60 mL/min, hepatic transaminases more than 2.5 times the upper limit of normal, cardiac ejection fraction less than 50%, or active serious infection. | ||
*Following lymphodepleting chemotherapy, YESCARTA was administered as a single intravenous infusion at a target dose of 2 × 106 CAR-positive viable T cells/kg (maximum permitted dose: 2 × 108 cells). The lymphodepleting regimen consisted of cyclophosphamide 500 mg/m2 intravenously and fludarabine 30 mg/m2 intravenously, both given on the fifth, fourth, and third day before YESCARTA. Bridging chemotherapy between leukapheresis and lymphodepleting chemotherapy was not permitted. All patients were hospitalized for YESCARTA infusion and for a minimum of 7 days afterward. | |||
( | *Of 111 patients who underwent leukapheresis, 101 received YESCARTA. Of the patients treated, the median age was 58 years (range: 23 to 76), 67% were male, and 89% were white. Most (76%) had DLBCL, 16% had transformed follicular lymphoma, and 8% had primary mediastinal large B-cell lymphoma. The median number of prior therapies was 3 (range: 1 to 10), 77% of the patients had refractory disease to a second or greater line of therapy, and 21% had relapsed within 1 year of autologous HSCT. | ||
*One out of 111 patients did not receive the product due to manufacturing failure. Nine other patients were not treated, primarily due to progressive disease or serious adverse reactions following leukapheresis. The median time from leukapheresis to product delivery was 17 days (range: 14 to 51 days), and the median time from leukapheresis to infusion was 24 days (range: 16 to 73 days). The median dose was 2.0 × 106 CAR-positive viable T cells/kg (range: 1.1 to 2.2 × 106 cells/kg). | |||
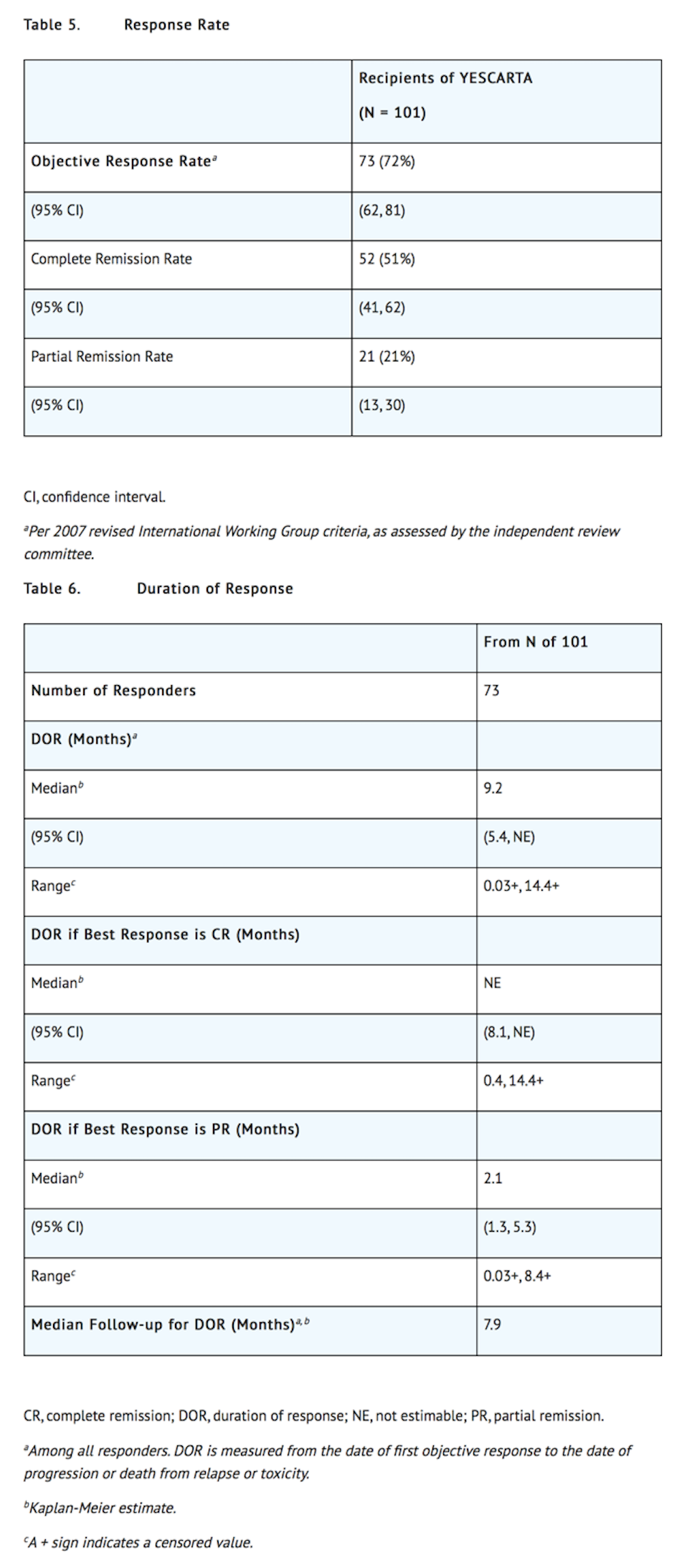
*Efficacy was established on the basis of complete remission (CR) rate and duration of response (DOR), as determined by an independent review committee (Table 5 and Table 6). The median time to response was 0.9 months (range: 0.8 to 6.2 months). Response durations were longer in patients who achieved CR, as compared to patients with a best response of partial remission (PR) (Table 6). Of the 52 patients who achieved CR, 14 initially had stable disease (7 patients) or PR (7 patients), with a median time to improvement of 2.1 months (range: 1.6 to 5.3 months). | |||
[[image:Axicabtagene_Ciloleucel_Clinical_Studies_Tables_1_and_2.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | |||
|howSupplied=*YESCARTA is supplied in an infusion bag (NDC 71287-119-01) containing approximately 68 mL of frozen suspension of genetically modified autologous T cells in 5% DMSO and 2.5% albumin (human). | |howSupplied=*YESCARTA is supplied in an infusion bag (NDC 71287-119-01) containing approximately 68 mL of frozen suspension of genetically modified autologous T cells in 5% DMSO and 2.5% albumin (human). | ||
Revision as of 13:25, 29 June 2018
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Yashasvi Aryaputra[2];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
CYTOKINE RELEASE SYNDROME and NEUROLOGIC TOXICITIES
See full prescribing information for complete Boxed Warning.
|
Overview
Axicabtagene ciloleucel is a Acetylcholine release inhibitor, Adrenergic receptor agonist that is FDA approved for the (type of indication of drug) of a list of indications, separated by commas.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include a list of adverse reactions, separated by commas..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
- None
Warnings
|
CYTOKINE RELEASE SYNDROME and NEUROLOGIC TOXICITIES
See full prescribing information for complete Boxed Warning.
|
Conidition 1
(Description)
Conidition 2
(Description)
Conidition 3
(Description)
Adverse Reactions
Clinical Trials Experience
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Condition 2
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Postmarketing Experience
(Description)
Drug Interactions
- Drug 1
- Drug 2
- Drug 3
- Drug 4
- Drug 5
Drug 1
(Description)
Drug 2
(Description)
Drug 3
(Description)
Drug 4
(Description)
Drug 5
(Description)
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
(Description)
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Axicabtagene ciloleucel in women who are pregnant.
Labor and Delivery
(Description)
Nursing Mothers
(Description)g
Pediatric Use
(Description)
Geriatic Use
(Description)
Gender
(Description)
Race
(Description)
Renal Impairment
(Description)
Hepatic Impairment
(Description)
Females of Reproductive Potential and Males
(Description)
Immunocompromised Patients
(Description)
Others
(Description)
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
There is limited information regarding the compatibility of Axicabtagene ciloleucel and IV administrations.
Overdosage
Acute Overdose
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description)
Pharmacology
Axicabtagene ciloleucel
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
(Description)
Structure
(Description with picture)
Pharmacodynamics
(Description)
Pharmacokinetics
(Description)
Nonclinical Toxicology
(Description)
Clinical Studies
Relapsed or Refractory Large B-Cell Lymphoma
- A single-arm, open-label, multicenter trial evaluated the efficacy of a single infusion of YESCARTA in adult patients with relapsed or refractory aggressive B-cell non-Hodgkin lymphoma. Eligible patients had refractory disease to the most recent therapy or relapse within 1 year after autologous hematopoietic stem cell transplantation (HSCT). The study excluded patients with prior allogeneic HSCT, any history of central nervous system lymphoma, ECOG performance status of 2 or greater, absolute lymphocyte count less than 100/µL, creatinine clearance less than 60 mL/min, hepatic transaminases more than 2.5 times the upper limit of normal, cardiac ejection fraction less than 50%, or active serious infection.
- Following lymphodepleting chemotherapy, YESCARTA was administered as a single intravenous infusion at a target dose of 2 × 106 CAR-positive viable T cells/kg (maximum permitted dose: 2 × 108 cells). The lymphodepleting regimen consisted of cyclophosphamide 500 mg/m2 intravenously and fludarabine 30 mg/m2 intravenously, both given on the fifth, fourth, and third day before YESCARTA. Bridging chemotherapy between leukapheresis and lymphodepleting chemotherapy was not permitted. All patients were hospitalized for YESCARTA infusion and for a minimum of 7 days afterward.
- Of 111 patients who underwent leukapheresis, 101 received YESCARTA. Of the patients treated, the median age was 58 years (range: 23 to 76), 67% were male, and 89% were white. Most (76%) had DLBCL, 16% had transformed follicular lymphoma, and 8% had primary mediastinal large B-cell lymphoma. The median number of prior therapies was 3 (range: 1 to 10), 77% of the patients had refractory disease to a second or greater line of therapy, and 21% had relapsed within 1 year of autologous HSCT.
- One out of 111 patients did not receive the product due to manufacturing failure. Nine other patients were not treated, primarily due to progressive disease or serious adverse reactions following leukapheresis. The median time from leukapheresis to product delivery was 17 days (range: 14 to 51 days), and the median time from leukapheresis to infusion was 24 days (range: 16 to 73 days). The median dose was 2.0 × 106 CAR-positive viable T cells/kg (range: 1.1 to 2.2 × 106 cells/kg).
- Efficacy was established on the basis of complete remission (CR) rate and duration of response (DOR), as determined by an independent review committee (Table 5 and Table 6). The median time to response was 0.9 months (range: 0.8 to 6.2 months). Response durations were longer in patients who achieved CR, as compared to patients with a best response of partial remission (PR) (Table 6). Of the 52 patients who achieved CR, 14 initially had stable disease (7 patients) or PR (7 patients), with a median time to improvement of 2.1 months (range: 1.6 to 5.3 months).
How Supplied
- YESCARTA is supplied in an infusion bag (NDC 71287-119-01) containing approximately 68 mL of frozen suspension of genetically modified autologous T cells in 5% DMSO and 2.5% albumin (human).
Storage
- Each YESCARTA infusion bag is individually packed in a metal cassette (NDC 71287-119-02). YESCARTA is stored in the vapor phase of liquid nitrogen and supplied in a liquid nitrogen dry shipper.
- Match the identity of the patient with the patient identifiers on the cassette and infusion bag upon receipt.
- Store YESCARTA frozen in the vapor phase of liquid nitrogen (less than or equal to minus 150ºC).
- Thaw before using.
Images
Drug Images
{{#ask: Page Name::Axicabtagene ciloleucel |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Axicabtagene ciloleucel |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Ensure that patients understand the risk of manufacturing failure (1% in clinical trial). In case of a manufacturing failure, a second manufacturing of YESCARTA may be attempted. In addition, while the patient awaits the product, additional chemotherapy (not the lymphodepletion) may be necessary and may increase the risk of adverse events during the pre-infusion period.
- Advise patients to seek immediate attention for any of the following:
- Cytokine Release Syndrome (CRS) - Signs or symptoms associated with CRS including fever, chills, fatigue, tachycardia, nausea, hypoxia, and hypotension.
- Neurologic Toxicities – Signs or symptoms associated with neurologic events including encephalopathy, seizures, changes in level of consciousness, speech disorders, tremors, and confusion.
- Serious Infections - Signs or symptoms associated with infection.
- Prolonged Cytopenia - Signs or symptoms associated with bone marrow suppression including neutropenia, anemia, thrombocytopenia, or febrile neutropenia.
- Advise patients for the need to:
- Refrain from driving or operating heavy or potentially dangerous machinery after YESCARTA infusion until at least 8 weeks after infusion.
- Have periodic monitoring of blood counts.
- Contact Kite at 1-844-454-KITE (5483) if they are diagnosed with a secondary malignancy.
Precautions with Alcohol
Alcohol-Axicabtagene ciloleucel interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Yescarta
Look-Alike Drug Names
There is limited information regarding Axicabtagene ciloleucel Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.