Lassa fever causes: Difference between revisions
Matt Pijoan (talk | contribs) No edit summary |
Matt Pijoan (talk | contribs) No edit summary |
||
| Line 29: | Line 29: | ||
* The Arenaviridae are a family of [[viruses]] whose members are generally associated with rodent-transmitted diseases in [[humans]]. Each [[virus]] usually is associated with a particular [[rodent]] host species in which it is maintained. [[Arenavirus]] infections are relatively common in humans in some areas of the world and can cause severe [[illnesses]]. | * The Arenaviridae are a family of [[viruses]] whose members are generally associated with rodent-transmitted diseases in [[humans]]. Each [[virus]] usually is associated with a particular [[rodent]] host species in which it is maintained. [[Arenavirus]] infections are relatively common in humans in some areas of the world and can cause severe [[illnesses]]. | ||
* The [[virus]] particles are [[spherical]] and have an average diameter of 110-130 nanometers. All are enveloped in a [[lipid]] (fat) membrane. Viewed in cross-section, they show grainy particles that are [[ribosomes]] acquired from their [[host]] cells. It is this characteristic that gave them their name, derived from the Latin "arena", which means "sandy". Their [[genome]], or genetic material, is composed of [[RNA]] only, and while their replication strategy is not completely understood, we know that new viral particles, called [[virions]], are created by [[budding]] from the surface of their [[hosts]]' cells. | * The [[virus]] particles are [[spherical]] and have an average diameter of 110-130 nanometers. All are enveloped in a [[lipid]] (fat) membrane. Viewed in cross-section, they show grainy particles that are [[ribosomes]] acquired from their [[host]] cells. It is this characteristic that gave them their name, derived from the Latin "arena", which means "sandy". Their [[genome]], or genetic material, is composed of [[RNA]] only, and while their replication strategy is not completely understood, we know that new viral particles, called [[virions]], are created by [[budding]] from the surface of their [[hosts]]' cells. | ||
<div style=" width:100%;">[[File:Lassa fever micro.png|left|thumb|500px|Outbreak Distribution Map Lassa Fever CDC.png<SMALL><SMALL>''[http://www.cdc.gov/vhf/virus-families/arenaviridae.html]''<ref name="CDC">{{Cite web | title = Center for Disease Control and Prevention (CDC) | url = http://www.cdc.gov}}</ref></SMALL></SMALL>]]</div> | <div style="float:left; width:100%;">[[File:Lassa fever micro.png|left|thumb|500px|Outbreak Distribution Map Lassa Fever CDC.png<SMALL><SMALL>''[http://www.cdc.gov/vhf/virus-families/arenaviridae.html]''<ref name="CDC">{{Cite web | title = Center for Disease Control and Prevention (CDC) | url = http://www.cdc.gov}}</ref></SMALL></SMALL>]]</div> | ||
===History of Arenaviridae=== | ===History of Arenaviridae=== | ||
Revision as of 18:19, 5 June 2015
| style="background:#Template:Taxobox colour;"|Lassa Virus (LASV) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
 | ||||||||||
| style="background:#Template:Taxobox colour;" | Virus classification | ||||||||||
|
|
Lassa fever Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Lassa fever causes On the Web |
|
American Roentgen Ray Society Images of Lassa fever causes |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [3]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [4]
Synonyms and keywords: Lassa hemorrhagic fever; LHF
Overview
Lassa fever is caused by the Lassa virus, a member of the Arenaviridae family. It is an enveloped, single-stranded, bisegmented RNA virus. Mastomysrodents shed the virus in urine and droppings. The direct contact with these materials or ingestion or inhalation, can lead to infection. Lassa virus enters the cell by the receptor-mediated endocytosis and undergoes very rapid replication and manifest the disease.
Virus
Taxonomy
Viruses; ssRNA viruses; ssRNA negative-strand viruses; Arenaviridae; Mammarenavirus [1]
- Lassa virus belongs to Arenaviridae [2].
- The Arenaviridae are a family of viruses whose members are generally associated with rodent-transmitted diseases in humans. Each virus usually is associated with a particular rodent host species in which it is maintained. Arenavirus infections are relatively common in humans in some areas of the world and can cause severe illnesses.
- The virus particles are spherical and have an average diameter of 110-130 nanometers. All are enveloped in a lipid (fat) membrane. Viewed in cross-section, they show grainy particles that are ribosomes acquired from their host cells. It is this characteristic that gave them their name, derived from the Latin "arena", which means "sandy". Their genome, or genetic material, is composed of RNA only, and while their replication strategy is not completely understood, we know that new viral particles, called virions, are created by budding from the surface of their hosts' cells.
- The first Arenavirus, Lymphocytic choriomeningitis virus (LCMV), was isolated in 1933 during a study of an epidemic of St. Louis encephalitis. Although not the cause of the outbreak, LCMV was found to be a cause of aseptic (nonbacterial) meningitis. By the 1960s, several similar viruses had been discovered and they were classified into the new family Arenaviridae. Since Tacaribe virus was found in 1956, new Arenavirus have been discovered on the average of every one to three years. A number of Arenavirus have been isolated in rodents only, but few cause hemorrhagic disease. Junin virus, isolated in 1958, was the first of these to be recognized. This virus causes Argentine hemorrhagic fever in a limited agrigultural area of the pampas in Argentina. Several years later, in 1963, in the remote savannas of the Beni province of Bolivia, Machupo virus was isolated. The next member of the virus family to be associated with an outbreak of human illness was Lassa virus in Nigeria in 1969. The most recent additions to these human pathogenic viruses were Guanarito detected in Venezuela in 1989, Sabia in Brazil in 1993, Chapare in Bolivia in 2004, and Lujo in South Africa in 2008.
Structure and genome
- Lassa viruses are enveloped, single-stranded, bisegmented, ambisense RNA viruses. Their genome[4] is contained in two RNA segments that code for two proteins each, one in each sense, for a total of four viral proteins.[5] The large segment encodes a small zinc-binding protein (Z) that regulates transcription and replication,[6][7] and the RNA polymerase (L). The small segment encodes the nucleoprotein (NP) and the surface glycoprotein precursor (GP, also known as the viral spike), which is proteolytically cleaved into the envelope glycoproteins GP1 and GP2 that bind to the alpha-dystroglycan receptor and mediate host cell entry.[8]
- Lassa fever causes hemorrhagic fever frequently shown by immunosuppression. Replication for Lassa virus is very rapid, while also demonstrating temporal control in replication.[9] The first replication step is transcription of mRNA copies of the negative- or minus-sense genome. This ensures an adequate supply of viral proteins for subsequent steps of replication, as the NP and L proteins are translated from the mRNA. The positive- or plus-sense genome, then makes viral complementary RNA (vcRNA)copies of itself. The RNA copies are a template for producing negative-sense progeny, but mRNA is also synthesized from it. The mRNA synthesized from vcRNA are translated to make the GP and Z proteins. This temporal control allows the spike proteins to be produced last, and therefore, delay recognition by the host immune system.
- Nucleotide studies of the genome have shown that Lassa has four lineages: three found in Nigeria and the fourth in Guinea, Liberia, and Sierra Leone. The Nigerian strains seem likely to have been ancestral to the others but additional work is required to confirm this.[10] One book that explains about this disease is The Lassa Ward by Ross I. Donaldson. He describes what it is like being a doctor and taking care of the Sierra Leone people who have contracted the virus.

Receptors
- The Lassa virus gains entry into the host cell by means of the cell-surface receptor the alpha-dystroglycan (alpha-DG),[10] a versatile receptor for proteins of the extracellular matrix. It shares this receptor with the prototypic Old World arenavirus lymphocytic choriomeningitis virus. Receptor recognition depends on a specific sugar modification of alpha-dystroglycan by a group of glycosyltransferases known as the LARGE proteins. Specific variants of the genes encoding these proteins appear to be under positive selection inWest Africa where Lassa is endemic. Alpha-dystroglycan is also used as a receptor by viruses of the New World clade C arenaviruses (Oliveros and Latino viruses). In contrast, the New World arenaviruses of clades A and B, which include the important viruses Machupo, Guanarito, Junin, and Sabia in addition to the non pathogenic Amapari virus, use the transferrin receptor 1. A small aliphatic amino acid at the GP1 glycoprotein amino acid position 260 is required for high-affinity binding to alpha-DG. In addition, GP1 amino acid position 259 also appears to be important, since all arenaviruses showing high-affinity alpha-DG binding possess a bulky aromatic amino acid (tyrosine or phenylalanine) at this position.
- Unlike most enveloped viruses which use clathrin coated pits for cellular entry and bind to their receptors in a pH dependent fashion, Lassa and lymphocytic choriomeningitis virus instead use an endocytotic pathway independent of clathrin, caveolin, dynamin and actin. Once within the cell the viruses are rapidly delivered to endosomes via vesicular trafficking albeit one that is largely independent of the small GTPases Rab5 and Rab7. On contact with the endosome pH-dependent membrane fusion occurs mediated by the envelope glycoprotein, which at the lower pH of the endosome binds the lysosome protein LAMP1 which results in membrane fusion and escape from the endosome.
Life cycle
- The life cycle of Lassa virus is similar to the Old World arenaviruses[11]. Lassa virus enters the cell by the receptor-mediated endocytosis. Which endocytotic pathway is used is not known yet, but at least the cellular entry is sensitive to cholesterol depletion. It was reported that virus internalization is limited upon cholesterol depletion. The receptor used for cell entry is alpha-dystroglycan, a highly conserved and ubiquitously expressed cell surface receptor for extracellular matrix proteins.
- Dystroglycan, which is later cleaved into alpha-dystroglycan and beta-dystroglycan is originally expressed in most cells to mature tissues, and it provides molecular link between the ECM and the actin-based cytoskeleton[12]. After virus enters the cell by alpha-dystroglycan mediated endocytosis, low-pH environment triggers pH-dependent membrane fusion and releases RNP (viral ribonucleoprotein) complex into the cytoplasm. Viral RNA is unpacked, and replication and transcription initiate in the cytoplasm.[12] As the replication starts, both S and L RNA genomes synthesize the antigenomic S and L RNAs, and from the antigenomic RNAs, genomic S and L RNA are synthesized. Both genomic and antigenomic RNAs are needed for transcription and translation. S RNA encodes GP and NP (viral nucleocapsid protein) proteins, and L RNA encodes Z and L proteins. L protein most likely represents the viral RNA-dependent RNA polymerase.[13] When the cell is infected by the virus, L polymerase is associated with the viral RNP and initiates the transcription of the genomic RNA. The 5’ and 3’ terminal 19 nt viral promoter regions of both RNA segments are necessary for recognition and binding of the viral polymerase. The primary transcription first transcribes mRNAs from the genomic S and L RNAs, which code NP and L proteins, respectively. Transcription terminates at the stem-loop (SL) structure within the intergenomic region. Arenaviruses use a cap snatching strategy to gain the cap structures from the cellular mRNAs, and it is mediated by the endonuclease activity of the L polymerase and the cap binding activity of NP. Antigenomic RNA transcribes viral genes GPC and Z, encoded in genomic orientation, from S and L segments respectively. The antigenomic RNA also serves as the template for the replication.[14] After translation of GPC, it is posttranslationally modified in the endoplasmic reticulum. GPC is cleaved into GP1 and GP2 at the later stage of the secretory pathway. It is reported the cellular protease SKI-1/S1P was responsible for the cleavage. Cleaved glycoproteins are incorporated into the virion envelope when the virus buds and release from the cell membrane.[13]
Vector
- The reservoir, or host, of Lassa virus is a rodent known as the "multimammate rat" (Mastomys natalensis). Once infected, this rodent is able to excrete virus in urine for an extended time period, maybe for the rest of its life. Mastomys rodents breed frequently, produce large numbers of offspring, and are numerous in the savannas and forests of west, central, and east Africa. In addition, Mastomys readily colonize human homes and areas where food is stored. All of these factors contribute to the relatively efficient spread of Lassa virus from infected rodents to humans.
- Transmission of Lassa virus to humans occurs most commonly through ingestion or inhalation. Mastomysrodents shed the virus in urine and droppings and direct contact with these materials, through touching soiled objects, eating contaminated food, or exposure to open cuts or sores, can lead to infection.
- Because Mastomys rodents often live in and around homes and scavenge on leftover human food items or poorly stored food, direct contact transmission is common. Mastomys rodents are sometimes consumed as a food source and infection may occur when rodents are caught and prepared. Contact with the virus may also occur when a person inhales tiny particles in the air contaminated with infected rodent excretions. This aerosol or airborne transmission may occur during cleaning activities, such as sweeping.
- Direct contact with infected rodents is not the only way in which people are infected; person-to-person transmission may occur after exposure to virus in the blood, tissue, secretions, or excretions of a Lassa virus-infected individual. Casual contact (including skin-to-skin contact without exchange of body fluids) does not spread Lassa virus. Person-to-person transmission is common in health care settings (called nosocomial transmission) where proper personal protective equipment (PPE) is not available or not used. Lassa virus may be spread in contaminated medical equipment, such as reused needles.
Microscopic Pathology
The images below display key features of the Lassa virus.
-
This photomicrograph shows hepatitis caused by the Lassa virus, using toluidine-blue azure II stain. Source: CDC microbiologist Dr. W. Winn.Adapted from Public Health Image Library (PHIL), Centers for Disease Control and Prevention.[15]
-
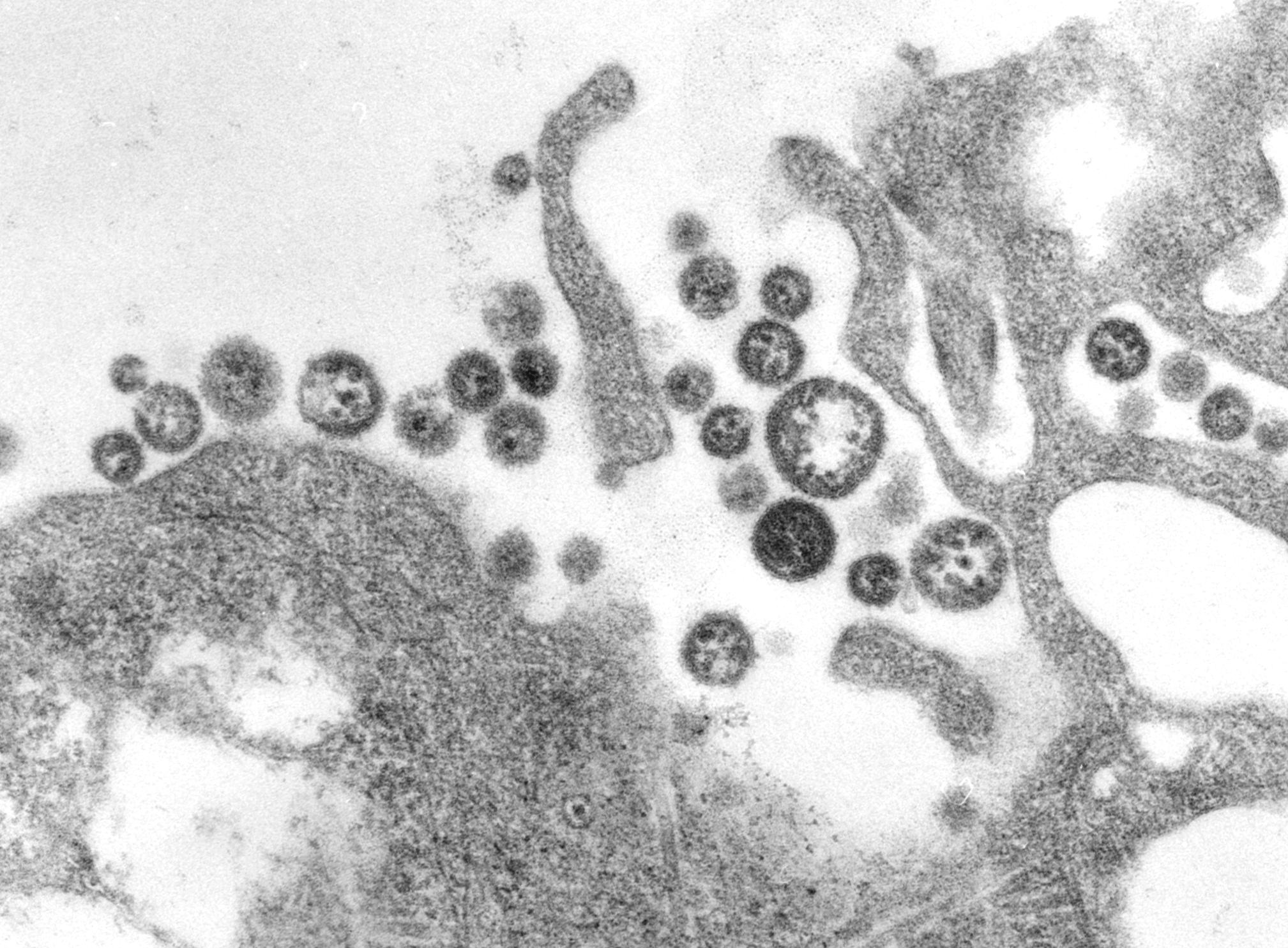
This transmission electron micrograph (TEM) demonstrates the ultrastructural morphologic changes in this tissue sample isolate.Adapted from Public Health Image Library (PHIL), Centers for Disease Control and Prevention.[15]
-
Scanning electron micrograph (SEM) revealing ultrastructural morphologic features of the Ebola virus from the Ivory Coast of Africa.Adapted from Public Health Image Library (PHIL), Centers for Disease Control and Prevention.[15]
-
Negatively-stained transmission electron micrograph (TEM) demonstrating the ultrastructural curvilinear morphologic features displayed by the Ebola virus from the Ivory Coast of Africa.Adapted from Public Health Image Library (PHIL), Centers for Disease Control and Prevention.[15]
References
- ↑ "Taxonomy browser (Lassavirus)".
- ↑ "The Centers for Disease Control and Prevention".
- ↑ 3.0 3.1 "Center for Disease Control and Prevention (CDC)".
- ↑ "Genome:The autobiography of a species in 23 chapters". Nat Genet. 24 (1): 21. 2000. doi:10.1038/71638. PMID 10615121.
- ↑ Moshkoff DA, Salvato MS, Lukashevich IS (2007). "Molecular characterization of a reassortant virus derived from Lassa and Mopeia viruses". Virus Genes. 34 (2): 169–76. doi:10.1007/s11262-006-0050-3. PMC 1892610. PMID 17143722.
- ↑ Cornu TI, de la Torre JC (2001). "RING finger Z protein of lymphocytic choriomeningitis virus (LCMV) inhibits transcription and RNA replication of an LCMV S-segment minigenome". J Virol. 75 (19): 9415–26. doi:10.1128/JVI.75.19.9415-9426.2001. PMC 114509. PMID 11533204.
- ↑ Djavani M, Lukashevich IS, Sanchez A, Nichol ST, Salvato MS (1997). "Completion of the Lassa fever virus sequence and identification of a RING finger open reading frame at the L RNA 5' End". Virology. 235 (2): 414–8. doi:10.1006/viro.1997.8722. PMID 9281522.
- ↑ Smelt SC, Borrow P, Kunz S, Cao W, Tishon A, Lewicki H; et al. (2001). "Differences in affinity of binding of lymphocytic choriomeningitis virus strains to the cellular receptor alpha-dystroglycan correlate with viral tropism and disease kinetics". J Virol. 75 (1): 448–57. doi:10.1128/JVI.75.1.448-457.2001. PMC 113937. PMID 11119613.
- ↑ Lashley FR (2006). "Emerging infectious diseases at the beginning of the 21st century". Online J Issues Nurs. 11 (1): 2. PMID 16629503.
- ↑ 10.0 10.1 Bowen MD, Rollin PE, Ksiazek TG, Hustad HL, Bausch DG, Demby AH; et al. (2000). "Genetic diversity among Lassa virus strains". J Virol. 74 (15): 6992–7004. PMC 112216. PMID 10888638.
- ↑ "Wikipedia lassa virus".
- ↑ 12.0 12.1 Rojek JM, Kunz S (2008). "Cell entry by human pathogenic arenaviruses". Cell Microbiol. 10 (4): 828–35. doi:10.1111/j.1462-5822.2007.01113.x. PMID 18182084.
- ↑ 13.0 13.1 Drosten C, Kümmerer BM, Schmitz H, Günther S (2003). "Molecular diagnostics of [[viral hemorrhagic fevers]]". Antiviral Res. 57 (1–2): 61–87. PMID 12615304. URL–wikilink conflict (help)
- ↑ Yun NE, Walker DH (2012). "Pathogenesis of Lassa fever". Viruses. 4 (10): 2031–48. doi:10.3390/v4102031. PMC 3497040. PMID 23202452.
- ↑ 15.0 15.1 15.2 15.3 "Public Health Image Library (PHIL), Centers for Disease Control and Prevention".

![This photomicrograph shows hepatitis caused by the Lassa virus, using toluidine-blue azure II stain. Source: CDC microbiologist Dr. W. Winn.Adapted from Public Health Image Library (PHIL), Centers for Disease Control and Prevention.[15]](/images/8/89/PHIL_2994_lores.jpg)
![This transmission electron micrograph (TEM) demonstrates the ultrastructural morphologic changes in this tissue sample isolate.Adapted from Public Health Image Library (PHIL), Centers for Disease Control and Prevention.[15]](/images/7/78/Ebola_virus1.png)
![Scanning electron micrograph (SEM) revealing ultrastructural morphologic features of the Ebola virus from the Ivory Coast of Africa.Adapted from Public Health Image Library (PHIL), Centers for Disease Control and Prevention.[15]](/images/c/c4/Ebola_virus3.png)
![Negatively-stained transmission electron micrograph (TEM) demonstrating the ultrastructural curvilinear morphologic features displayed by the Ebola virus from the Ivory Coast of Africa.Adapted from Public Health Image Library (PHIL), Centers for Disease Control and Prevention.[15]](/images/2/2e/Ebola_virus4.png)