Norethindrone: Difference between revisions
No edit summary |
No edit summary |
||
| Line 46: | Line 46: | ||
|fdaLIADAdult= | |fdaLIADAdult= | ||
===== | =====Prevention of Pregnancy===== | ||
* | *To achieve maximum contraceptive effectiveness, norethindrone tablets must be taken exactly as directed. One tablet is taken every day, at the same time. Administration is continuous, with no interruption between pill packs. See DETAILED PATIENT LABELING for detailed instruction. | ||
:* | *Efficacy | ||
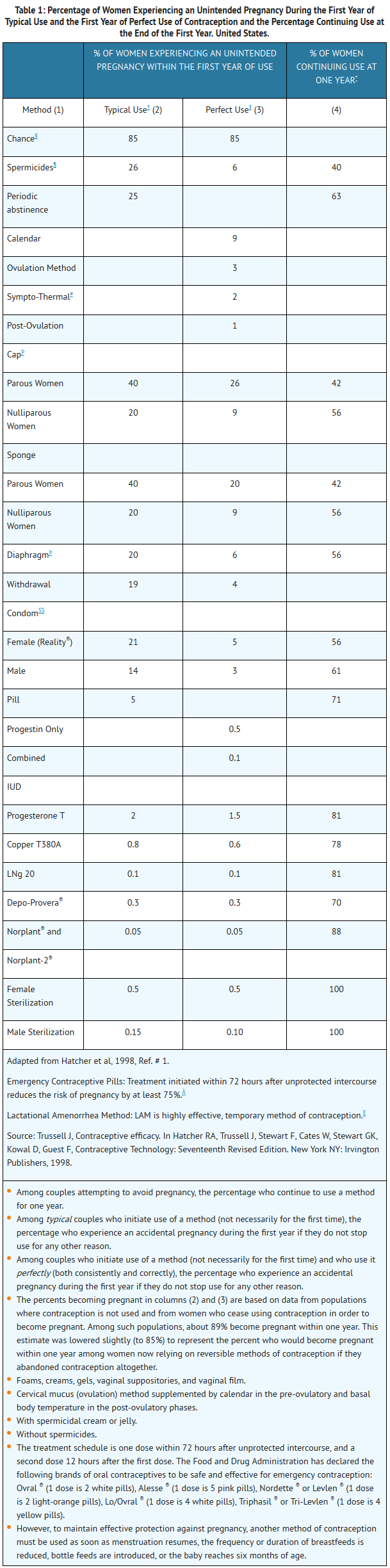
:*If used perfectly, the first-year failure rate for progestin-only oral contraceptives is 0.5%. However, the typical failure rate is estimated to be closer to 5%, due to late or omitted pills. Table 1 lists the pregnancy rates for users of all major methods of contraception. | |||
t1 | |||
<!--Off-Label Use and Dosage (Adult)--> | <!--Off-Label Use and Dosage (Adult)--> | ||
| Line 111: | Line 102: | ||
|fdaLIADPed= | |fdaLIADPed= | ||
There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | ||
| Line 127: | Line 110: | ||
|offLabelPedGuideSupport= | |offLabelPedGuideSupport= | ||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ||
| Line 147: | Line 116: | ||
|offLabelPedNoGuideSupport= | |offLabelPedNoGuideSupport= | ||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ||
| Line 162: | Line 123: | ||
|contraindications= | |contraindications= | ||
* | *Known or suspected [[pregnancy]] | ||
*Known or suspected [[carcinoma of the breast]] | |||
*Undiagnosed abnormal [[genital bleeding]] | |||
*[[Hypersensitivity]] to any component of this product | |||
*[[Benign]] or [[malignant]] [[liver tumors]] | |||
* | *[[Acute liver disease]] | ||
<!--Warnings--> | |||
|warnings= | |||
*Cigarette smoking increases the risk of serious [[cardiovascular]] disease. Women who use oral contraceptives should be strongly advised not to smoke. | |||
*Norethindrone tablets do not contain [[estrogen]] and, therefore, this insert does not discuss the serious health risks that have been associated with the [[estrogen]] component of combined [[oral contraceptives]] (COCs). The healthcare professional is referred to the prescribing information of combined [[oral contraceptives]] for a discussion of those risks. The relationship between [[progestin]]-only oral contraceptives and these risks is not fully defined. The healthcare professional should remain alert to the earliest manifestation of symptoms of any serious disease and discontinue oral contraceptive therapy when appropriate. | |||
*[[Ectopic Pregnancy]] | |||
:*The incidence of ectopic pregnancies for [[progestin]]-only oral contraceptive users is 5 per 1000 woman-years. Up to 10% of pregnancies reported in clinical studies of [[progestin]]-only [[oral contraceptive]] users are [[extrauterine]]. Although symptoms of ectopic pregnancy should be watched for, a history of [[ectopic pregnancy]] need not be considered a contraindication to use of this contraceptive method. Healthcare professionals should be alert to the possibility of an [[ectopic pregnancy]] in women who become pregnant or complain of [[lower abdominal pain]] while on [[progestin]]-only [[oral contraceptives.]] | |||
*Delayed Follicular Atresia/Ovarian Cysts | |||
:*If follicular development occurs, atresia of the follicle is sometimes delayed and the follicle may continue to grow beyond the size it would attain in a normal cycle. Generally these enlarged follicles disappear spontaneously. Often they are asymptomatic; in some cases they are associated with mild abdominal pain. Rarely they may twist or rupture, requiring surgical intervention. | |||
*Irregular Genital Bleeding | |||
:*Irregular menstrual patterns are common among women using progestin-only oral contraceptives. If genital bleeding is suggestive of infection, malignancy or other abnormal conditions, such nonpharmacologic causes should be ruled out. If prolonged amenorrhea occurs, the possibility of pregnancy should be evaluated. | |||
*Carcinoma of the Breast and Reproductive Organs | |||
:*Some epidemiological studies of oral contraceptive users have reported an increased relative risk of developing breast cancer, particularly at a younger age and apparently related to duration of use. These studies have predominantly involved combined oral contraceptives and there is insufficient data to determine whether the use of POPs similarly increases the risk. | |||
:*A meta-analysis of 54 studies found a small increase in the frequency of having breast cancer diagnosed for women who were currently using combined oral contraceptives or had used them within the past ten years. | |||
:*This increase in the frequency of breast cancer diagnosis, within ten years of stopping use, was generally accounted for by cancers localized to the breast. There was no increase in the frequency of having breast cancer diagnosed ten or more years after cessation of use. | |||
:*Women with breast cancer should not use oral contraceptives because the role of female hormones in breast cancer has not been fully determined. | |||
:*Some studies suggest that oral contraceptive use has been associated with an increase in the risk of cervical intraepithelial neoplasia in some populations of women. However, there continues to be controversy about the extent to which such findings may be due to differences in sexual behavior and other factors. There is insufficient data to determine whether the use of POPs increases the risk of developing cervical intraepithelial neoplasia. | |||
*Hepatic Neoplasia | |||
:*Benign hepatic adenomas are associated with combined oral contraceptive use, although the incidence of benign tumors is rare in the United States. Rupture of benign, hepatic adenomas may cause death through intra-abdominal hemorrhage. | |||
:*Studies have shown an increased risk of developing hepatocellular carcinoma in combined oral contraceptive users. However, these cancers are rare in the U.S. There is insufficient data to determine whether POPs increase the risk of developing hepatic neoplasia. | |||
====Precautions==== | |||
*General | |||
:*Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases. | |||
*Physical Examination and Follow-up | |||
:*It is considered good medical practice for sexually active women using oral contraceptives to have annual history and physical examinations. The physical examination may be deferred until after initiation of oral contraceptives if requested by the woman and judged appropriate by the healthcare professional. | |||
*Carbohydrate and Lipid Metabolism | |||
:*Some users may experience slight deterioration in glucose tolerance, with increases in plasma insulin but women with diabetes mellitus who use progestin-only oral contraceptives do not generally experience changes in their insulin requirements. Nonetheless, prediabetic and diabetic women in particular should be carefully monitored while taking POPs. | |||
:*Lipid metabolism is occasionally affected in that HDL, HDL2, and apolipoprotein A-I and A-II may be decreased; hepatic lipase may be increased. There is usually no effect on total cholesterol, HDL3, LDL, or VLDL. | |||
*Headache | |||
:*The onset or exacerbation of migraine or development of severe headache with focal neurological symptoms which is recurrent or persistent requires discontinuation of progestin-only contraceptives and evaluation of the cause. | |||
<!--Adverse Reactions--> | |||
<!--Clinical Trials Experience--> | |||
|clinicalTrials= | |||
*Adverse reactions reported with the use of POPs include: | |||
:*[[Menstrual]] irregularity is the most frequently reported side effect. | |||
:*Frequent and irregular bleeding are common, while long duration of bleeding episodes and [[amenorrhea]] are less likely. | |||
:*[[Headache]], [[breast]] tenderness, [[nausea]], and [[dizziness]] are increased among [[progestin]]-only [[oral contraceptive]] users in some studies. | |||
:*[[Androgenic]] side effects such as [[acne]], [[hirsutism]], and [[weight gain]] occur rarely. | |||
*The following adverse reactions were also reported in clinical trials or during post-marketing experience: | |||
=====Body as a Whole===== | |||
[[fatigue]], [[edema]] | |||
=====Digestive===== | |||
[[vomiting]], [[abdominal pain]], [[hepatitis]], [[jaundice]] cholestatic | |||
=====Musculoskeletal===== | =====Musculoskeletal===== | ||
[[pain]] in extremity | |||
=====Neurologic===== | =====Neurologic===== | ||
[[depression]], [[nervousness]] | |||
=====Skin and Hypersensitivy Reactions===== | =====Skin and Hypersensitivy Reactions===== | ||
[[anaphylactic]]/[[anaphylactoid]] reaction, [[hypersensitivity]], [[alopecia]], [[rash]], rash [[pruritic]]. | |||
=====Urogenital===== | =====Urogenital===== | ||
[[genital discharge]]; breast pain, [[menstruation]] delayed, suppressed [[lactation]], vaginal [[hemorrhage]], [[menorrhagia]], [[withdrawal bleed]] when product is stopped | |||
<!--Postmarketing Experience--> | <!--Postmarketing Experience--> | ||
| Line 252: | Line 222: | ||
There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | ||
<!--Drug Interactions--> | |||
|drugInteractions= | |||
*The effectiveness of [[progestin]]-only pills is reduced by [[hepatic]] enzyme-inducing drugs such as the [[anticonvulsants]] [[phenytoin]], [[carbamazepine]], and [[barbiturates]], and the [[antituberculosis]] drug [[rifampin]]. No significant interaction has been found with broad-spectrum antibiotics. | |||
*Herbal products containing St. John’s Wort (Hypericum perforatum) may induce [[hepatic]] enzymes (cytochrome P450) and p-glycoprotein transporter and may reduce the effectiveness of [[contraceptive]] [[steroids]]. This may also result in breakthrough [[bleeding]]. | |||
*Concurrent use of [[bosentan]] and norethindrone containing products may result in decreased concentrations of these [[contraceptive]] hormones thereby increasing the risk of unintended [[pregnancy]] and unscheduled bleeding. | |||
* | |||
<!--Use in Specific Populations--> | <!--Use in Specific Populations--> | ||
| Line 315: | Line 236: | ||
|useInPregnancyFDA= | |useInPregnancyFDA= | ||
* '''Pregnancy Category''' | * '''Pregnancy Category''' | ||
*Many studies have found no effects on fetal development associated with long-term use of contraceptive doses of oral progestins. The few studies of infant growth and development that have been conducted have not demonstrated significant adverse effects. It is nonetheless prudent to rule out suspected pregnancy before initiating any hormonal contraceptive use. | |||
|useInPregnancyAUS= | |useInPregnancyAUS= | ||
| Line 325: | Line 248: | ||
|useInNursing= | |useInNursing= | ||
*In general, no adverse effects have been found on breastfeeding performance or on the health, growth, or development of the infant. However, isolated post-marketing cases of decreased milk production have been reported. Small amounts of progestins pass into the breast milk of nursing mothers, resulting in detectable steroid levels in infant plasma. | |||
|useInPed= | |useInPed= | ||
*Safety and efficacy of norethindronetablets have been established in women of reproductive age. Safety and efficacy are expected to be the same for postpubertal adolescents under the age of 16 and for users 16 years and older. Use of this product before menarche is not indicated. | |||
|useInGeri= | |useInGeri= | ||
| Line 377: | Line 302: | ||
===Acute Overdose=== | ===Acute Overdose=== | ||
*There have been no reports of serious ill effects from overdosage, including ingestion by children. | |||
* | |||
===Chronic Overdose=== | ===Chronic Overdose=== | ||
| Line 401: | Line 320: | ||
|mechAction= | |mechAction= | ||
* | *Errin® progestin-only oral contraceptives prevent conception by suppressing ovulation in approximately half of users, thickening the cervical mucus to inhibit sperm penetration, lowering the midcycle LH and FSH peaks, slowing the movement of the ovum through the fallopian tubes, and altering the endometrium. | ||
<!--Structure--> | <!--Structure--> | ||
| Line 407: | Line 326: | ||
|structure= | |structure= | ||
* | *Norethindrone, USP is a white to creamy white, odorless, crystalline powder. It is stable in air. Practically insoluble in water; soluble in chloroform and in dioxane; sparingly soluble in alcohol; slightly soluble in ether. The chemical name for norethindrone is 17-Hydroxy-19-nor-17α-pregn-4-en-20-yn-3-one. The structural formula is as follows: | ||
: [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | : [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
*Each yellow tablet contains 0.35 mg norethindrone, USP and has the following inactive ingredients: anhydrous lactose, corn starch, D&C yellow no. 10 aluminum lake, ethylcellulose aqueous dispersion, lactose monohydrate, magnesium stearate, microcrystalline cellulose and povidone. | |||
*Meets USP Dissolution Test 2. | |||
<!--Pharmacodynamics--> | <!--Pharmacodynamics--> | ||
| Line 421: | Line 344: | ||
|PK= | |PK= | ||
There | *Serum progestin levels peak about two hours after oral administration, followed by rapid distribution and elimination. By 24 hours after drug ingestion, serum levels are near baseline, making efficacy dependent upon rigid adherence to the dosing schedule. There are large variations in serum levels among individual users. Progestin-only administration results in lower steady-state serum progestin levels and a shorter elimination half-life than concomitant administration with estrogens. | ||
<!--Nonclinical Toxicology--> | <!--Nonclinical Toxicology--> | ||
| Line 445: | Line 368: | ||
|fdaPatientInfo= | |fdaPatientInfo= | ||
*Counseling Issues | |||
*The following points should be discussed with prospective users before prescribing progestin-only oral contraceptives: | |||
:*The necessity of taking pills at the same time every day, including throughout all bleeding episodes. | |||
:*The need to use a backup method such as a condom and spermicide for the next 48 hours whenever a progestin-only oral contraceptive is taken 3 or more hours late. | |||
:*The potential side effects of progestin-only oral contraceptives, particularly menstrual irregularities. | |||
:*The need to inform the healthcare professional of prolonged episodes of bleeding, amenorrhea or severe abdominal pain. | |||
:*The importance of using a barrier method in addition to progestin-only oral contraceptives if a woman is at risk of contracting or transmitting STDs/HIV. | |||
1 | |||
2 | |||
<!--Precautions with Alcohol--> | <!--Precautions with Alcohol--> | ||
Revision as of 14:23, 16 October 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Title
See full prescribing information for complete Boxed Warning.
ConditionName:
|
Overview
Norethindrone is a that is FDA approved for the {{{indicationType}}} of . There is a Black Box Warning for this drug as shown here. Common adverse reactions include .
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Prevention of Pregnancy
- To achieve maximum contraceptive effectiveness, norethindrone tablets must be taken exactly as directed. One tablet is taken every day, at the same time. Administration is continuous, with no interruption between pill packs. See DETAILED PATIENT LABELING for detailed instruction.
- Efficacy
- If used perfectly, the first-year failure rate for progestin-only oral contraceptives is 0.5%. However, the typical failure rate is estimated to be closer to 5%, due to late or omitted pills. Table 1 lists the pregnancy rates for users of all major methods of contraception.
t1
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Norethindrone in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Norethindrone in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Norethindrone in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Norethindrone in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Norethindrone in pediatric patients.
Contraindications
- Known or suspected pregnancy
- Known or suspected carcinoma of the breast
- Undiagnosed abnormal genital bleeding
- Hypersensitivity to any component of this product
*Benign or malignant liver tumors
Warnings
|
Title
See full prescribing information for complete Boxed Warning.
ConditionName:
|
- Cigarette smoking increases the risk of serious cardiovascular disease. Women who use oral contraceptives should be strongly advised not to smoke.
- Norethindrone tablets do not contain estrogen and, therefore, this insert does not discuss the serious health risks that have been associated with the estrogen component of combined oral contraceptives (COCs). The healthcare professional is referred to the prescribing information of combined oral contraceptives for a discussion of those risks. The relationship between progestin-only oral contraceptives and these risks is not fully defined. The healthcare professional should remain alert to the earliest manifestation of symptoms of any serious disease and discontinue oral contraceptive therapy when appropriate.
- The incidence of ectopic pregnancies for progestin-only oral contraceptive users is 5 per 1000 woman-years. Up to 10% of pregnancies reported in clinical studies of progestin-only oral contraceptive users are extrauterine. Although symptoms of ectopic pregnancy should be watched for, a history of ectopic pregnancy need not be considered a contraindication to use of this contraceptive method. Healthcare professionals should be alert to the possibility of an ectopic pregnancy in women who become pregnant or complain of lower abdominal pain while on progestin-only oral contraceptives.
- Delayed Follicular Atresia/Ovarian Cysts
- If follicular development occurs, atresia of the follicle is sometimes delayed and the follicle may continue to grow beyond the size it would attain in a normal cycle. Generally these enlarged follicles disappear spontaneously. Often they are asymptomatic; in some cases they are associated with mild abdominal pain. Rarely they may twist or rupture, requiring surgical intervention.
- Irregular Genital Bleeding
- Irregular menstrual patterns are common among women using progestin-only oral contraceptives. If genital bleeding is suggestive of infection, malignancy or other abnormal conditions, such nonpharmacologic causes should be ruled out. If prolonged amenorrhea occurs, the possibility of pregnancy should be evaluated.
- Carcinoma of the Breast and Reproductive Organs
- Some epidemiological studies of oral contraceptive users have reported an increased relative risk of developing breast cancer, particularly at a younger age and apparently related to duration of use. These studies have predominantly involved combined oral contraceptives and there is insufficient data to determine whether the use of POPs similarly increases the risk.
- A meta-analysis of 54 studies found a small increase in the frequency of having breast cancer diagnosed for women who were currently using combined oral contraceptives or had used them within the past ten years.
- This increase in the frequency of breast cancer diagnosis, within ten years of stopping use, was generally accounted for by cancers localized to the breast. There was no increase in the frequency of having breast cancer diagnosed ten or more years after cessation of use.
- Women with breast cancer should not use oral contraceptives because the role of female hormones in breast cancer has not been fully determined.
- Some studies suggest that oral contraceptive use has been associated with an increase in the risk of cervical intraepithelial neoplasia in some populations of women. However, there continues to be controversy about the extent to which such findings may be due to differences in sexual behavior and other factors. There is insufficient data to determine whether the use of POPs increases the risk of developing cervical intraepithelial neoplasia.
- Hepatic Neoplasia
- Benign hepatic adenomas are associated with combined oral contraceptive use, although the incidence of benign tumors is rare in the United States. Rupture of benign, hepatic adenomas may cause death through intra-abdominal hemorrhage.
- Studies have shown an increased risk of developing hepatocellular carcinoma in combined oral contraceptive users. However, these cancers are rare in the U.S. There is insufficient data to determine whether POPs increase the risk of developing hepatic neoplasia.
Precautions
- General
- Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
- Physical Examination and Follow-up
- It is considered good medical practice for sexually active women using oral contraceptives to have annual history and physical examinations. The physical examination may be deferred until after initiation of oral contraceptives if requested by the woman and judged appropriate by the healthcare professional.
- Carbohydrate and Lipid Metabolism
- Some users may experience slight deterioration in glucose tolerance, with increases in plasma insulin but women with diabetes mellitus who use progestin-only oral contraceptives do not generally experience changes in their insulin requirements. Nonetheless, prediabetic and diabetic women in particular should be carefully monitored while taking POPs.
- Lipid metabolism is occasionally affected in that HDL, HDL2, and apolipoprotein A-I and A-II may be decreased; hepatic lipase may be increased. There is usually no effect on total cholesterol, HDL3, LDL, or VLDL.
- Headache
- The onset or exacerbation of migraine or development of severe headache with focal neurological symptoms which is recurrent or persistent requires discontinuation of progestin-only contraceptives and evaluation of the cause.
Adverse Reactions
Clinical Trials Experience
- Adverse reactions reported with the use of POPs include:
- Menstrual irregularity is the most frequently reported side effect.
- Frequent and irregular bleeding are common, while long duration of bleeding episodes and amenorrhea are less likely.
- Headache, breast tenderness, nausea, and dizziness are increased among progestin-only oral contraceptive users in some studies.
- Androgenic side effects such as acne, hirsutism, and weight gain occur rarely.
- The following adverse reactions were also reported in clinical trials or during post-marketing experience:
Body as a Whole
Digestive
vomiting, abdominal pain, hepatitis, jaundice cholestatic
Musculoskeletal
pain in extremity
Neurologic
Skin and Hypersensitivy Reactions
anaphylactic/anaphylactoid reaction, hypersensitivity, alopecia, rash, rash pruritic.
Urogenital
genital discharge; breast pain, menstruation delayed, suppressed lactation, vaginal hemorrhage, menorrhagia, withdrawal bleed when product is stopped
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Norethindrone in the drug label.
Drug Interactions
- The effectiveness of progestin-only pills is reduced by hepatic enzyme-inducing drugs such as the anticonvulsants phenytoin, carbamazepine, and barbiturates, and the antituberculosis drug rifampin. No significant interaction has been found with broad-spectrum antibiotics.
- Herbal products containing St. John’s Wort (Hypericum perforatum) may induce hepatic enzymes (cytochrome P450) and p-glycoprotein transporter and may reduce the effectiveness of contraceptive steroids. This may also result in breakthrough bleeding.
- Concurrent use of bosentan and norethindrone containing products may result in decreased concentrations of these contraceptive hormones thereby increasing the risk of unintended pregnancy and unscheduled bleeding.
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Many studies have found no effects on fetal development associated with long-term use of contraceptive doses of oral progestins. The few studies of infant growth and development that have been conducted have not demonstrated significant adverse effects. It is nonetheless prudent to rule out suspected pregnancy before initiating any hormonal contraceptive use.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Norethindrone in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Norethindrone during labor and delivery.
Nursing Mothers
- In general, no adverse effects have been found on breastfeeding performance or on the health, growth, or development of the infant. However, isolated post-marketing cases of decreased milk production have been reported. Small amounts of progestins pass into the breast milk of nursing mothers, resulting in detectable steroid levels in infant plasma.
Pediatric Use
- Safety and efficacy of norethindronetablets have been established in women of reproductive age. Safety and efficacy are expected to be the same for postpubertal adolescents under the age of 16 and for users 16 years and older. Use of this product before menarche is not indicated.
Geriatic Use
There is no FDA guidance on the use of Norethindrone with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Norethindrone with respect to specific gender populations.
Race
There is no FDA guidance on the use of Norethindrone with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Norethindrone in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Norethindrone in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Norethindrone in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Norethindrone in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Norethindrone in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Norethindrone in the drug label.
Overdosage
Acute Overdose
- There have been no reports of serious ill effects from overdosage, including ingestion by children.
Chronic Overdose
There is limited information regarding Chronic Overdose of Norethindrone in the drug label.
Pharmacology
There is limited information regarding Norethindrone Pharmacology in the drug label.
Mechanism of Action
- Errin® progestin-only oral contraceptives prevent conception by suppressing ovulation in approximately half of users, thickening the cervical mucus to inhibit sperm penetration, lowering the midcycle LH and FSH peaks, slowing the movement of the ovum through the fallopian tubes, and altering the endometrium.
Structure
- Norethindrone, USP is a white to creamy white, odorless, crystalline powder. It is stable in air. Practically insoluble in water; soluble in chloroform and in dioxane; sparingly soluble in alcohol; slightly soluble in ether. The chemical name for norethindrone is 17-Hydroxy-19-nor-17α-pregn-4-en-20-yn-3-one. The structural formula is as follows:
- Each yellow tablet contains 0.35 mg norethindrone, USP and has the following inactive ingredients: anhydrous lactose, corn starch, D&C yellow no. 10 aluminum lake, ethylcellulose aqueous dispersion, lactose monohydrate, magnesium stearate, microcrystalline cellulose and povidone.
- Meets USP Dissolution Test 2.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Norethindrone in the drug label.
Pharmacokinetics
- Serum progestin levels peak about two hours after oral administration, followed by rapid distribution and elimination. By 24 hours after drug ingestion, serum levels are near baseline, making efficacy dependent upon rigid adherence to the dosing schedule. There are large variations in serum levels among individual users. Progestin-only administration results in lower steady-state serum progestin levels and a shorter elimination half-life than concomitant administration with estrogens.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Norethindrone in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Norethindrone in the drug label.
How Supplied
Storage
There is limited information regarding Norethindrone Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Norethindrone |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Norethindrone |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Counseling Issues
- The following points should be discussed with prospective users before prescribing progestin-only oral contraceptives:
- The necessity of taking pills at the same time every day, including throughout all bleeding episodes.
- The need to use a backup method such as a condom and spermicide for the next 48 hours whenever a progestin-only oral contraceptive is taken 3 or more hours late.
- The potential side effects of progestin-only oral contraceptives, particularly menstrual irregularities.
- The need to inform the healthcare professional of prolonged episodes of bleeding, amenorrhea or severe abdominal pain.
- The importance of using a barrier method in addition to progestin-only oral contraceptives if a woman is at risk of contracting or transmitting STDs/HIV.
1
2
Precautions with Alcohol
- Alcohol-Norethindrone interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Norethindrone |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Norethindrone |Label Name=Norethindrone11.png
}}
{{#subobject:
|Label Page=Norethindrone |Label Name=Norethindrone11.png
}}