Pentoxifylline: Difference between revisions
No edit summary |
No edit summary |
||
| Line 112: | Line 112: | ||
The table indicates that in the tablet studies few patients discontinued because of adverse effects. | The table indicates that in the tablet studies few patients discontinued because of adverse effects. | ||
[[File:Pentoxifylline table 01.jpg|thumb|400px]] | [[File:Pentoxifylline table 01.jpg|thumb|400px|This image is provided by the National Library of Medicine.]] | ||
TRENTAL has been marketed in Europe and elsewhere since 1972. In addition to the above symptoms, the following have been reported spontaneously since marketing or occurred in other clinical trials with an incidence of less than 1%; the causal relationship was uncertain: | TRENTAL has been marketed in Europe and elsewhere since 1972. In addition to the above symptoms, the following have been reported spontaneously since marketing or occurred in other clinical trials with an incidence of less than 1%; the causal relationship was uncertain: | ||
| Line 153: | Line 153: | ||
The chemical structure is: | The chemical structure is: | ||
[[Pentoxifylline_table_02.jpg|thumb|400px]] | [[Pentoxifylline_table_02.jpg|thumb|400px|This image is provided by the National Library of Medicine.]] | ||
|PK=After oral administration in aqueous solution pentoxifylline is almost completely absorbed. It undergoes a first-pass effect and the various metabolites appear in plasma very soon after dosing. Peak plasma levels of the parent compound and its metabolites are reached within 1 hour. The major metabolites are Metabolite l (1-[5-hydroxyhexyl]-3,7-dimethylxanthine) and Metabolite V (1-[3-carboxypropyl]-3,7-dimethylxanthine), and plasma levels of these metabolites are 5 and 8 times greater, respectively, than pentoxifylline. | |PK=After oral administration in aqueous solution pentoxifylline is almost completely absorbed. It undergoes a first-pass effect and the various metabolites appear in plasma very soon after dosing. Peak plasma levels of the parent compound and its metabolites are reached within 1 hour. The major metabolites are Metabolite l (1-[5-hydroxyhexyl]-3,7-dimethylxanthine) and Metabolite V (1-[3-carboxypropyl]-3,7-dimethylxanthine), and plasma levels of these metabolites are 5 and 8 times greater, respectively, than pentoxifylline. | ||
Following oral administration of aqueous solutions containing 100 to 400 mg of pentoxifylline, the pharmacokinetics of the parent compound and Metabolite l are dose-related and not proportional (non-linear), with half-life and area under the blood-level time curve (AUC) increasing with dose. The elimination kinetics of Metabolite V are not dose-dependent. The apparent plasma half-life of pentoxifylline varies from 0.4 to 0.8 hours and the apparent plasma half-lives of its metabolites vary from 1 to 1.6 hours. There is no evidence of accumulation or enzyme induction (Cytochrome P450) following multiple oral doses. | Following oral administration of aqueous solutions containing 100 to 400 mg of pentoxifylline, the pharmacokinetics of the parent compound and Metabolite l are dose-related and not proportional (non-linear), with half-life and area under the blood-level time curve (AUC) increasing with dose. The elimination kinetics of Metabolite V are not dose-dependent. The apparent plasma half-life of pentoxifylline varies from 0.4 to 0.8 hours and the apparent plasma half-lives of its metabolites vary from 1 to 1.6 hours. There is no evidence of accumulation or enzyme induction (Cytochrome P450) following multiple oral doses. | ||
Revision as of 15:10, 16 June 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Pentoxifylline is a Methylxanthine that is FDA approved for the {{{indicationType}}} of treatment of patients with intermittent claudication on the basis of chronic occlusive arterial disease of the limbs. Common adverse reactions include nausea , vomiting.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Intermittent Claudication
- Dosing Information
- Dosing information: 400 mg PO tid with meals
- Duration: 8 weeks
- Digestive and central nervous system side effects are dose related. If patients develop these effects it is recommended that the dosage be lowered to one tablet twice a day (800 mg/day). If side effects persist at this lower dosage, the administration of Pentoxifylline Extended-release Tablet should be discontinued.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Pentoxifylline in adult patients.
Non–Guideline-Supported Use
Alcoholic hepatitis
- Dosing information
Cerebrovascular disease
- Dosing information
Congestive heart failure
- Dosing information
Adjunct treatment in the Diabetes mellitus
- Dosing information
- 400 mg PO tid [10]
Proteinuria in the Diabetes mellitus
- Dosing information
Gangrenous disorder
- Dosing information
- 400 mg PO tid [13]
Male infertility
- Dosing information
- 800-1200 mg/day for 3 months[14]
Peyronie's disease, Early chronic
- Dosing information
- 400 mg PO bid [15]
Retinal vascular occlusion
- Dosing information
Rheumatoid arthritis
- Dosing information
- 400 mg PO tid
Sepsis
- Dosing information
- 400 mg PO tid [18]
Prophylaxis of Thromboembolic disorder; Prophylaxis
- Dosing information
Prophylaxis of Thromboembolic disorder; Prophylaxis
- Dosing information
Vasculitis
- Dosing information
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Pentoxifylline in pediatric patients.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of Pentoxifylline in pediatric patients.
Contraindications
TRENTAL should not be used in patients with recent cerebral and/or retinal hemorrhage or in patients who have previously exhibited intolerance to this product or methylxanthines such as caffeine, theophylline, and theobromine.
Warnings
At the first sign of anaphylactic/anaphylactoid reaction, TRENTAL must be discontinued. Patients with chronic occlusive arterial disease of the limbs frequently show other manifestations of arteriosclerotic disease. TRENTAL has been used safely for treatment of peripheral arterial disease in patients with concurrent coronary artery and cerebrovascular diseases, but there have been occasional reports of angina, hypotension, and arrhythmia. Controlled trials do not show that TRENTAL causes such adverse effects more often than placebo, but, as it is a methylxanthine derivative, it is possible some individuals will experience such responses. Patients on Warfarin should have more frequent monitoring of prothrombin times, while patients with other risk factors complicated by hemorrhage (e.g., recent surgery, peptic ulceration, cerebral and/or retinal bleeding) should have periodic examinations for bleeding including, hematocrit and/or hemoglobin.
Adverse Reactions
Clinical Trials Experience
Clinical trials were conducted using either extended-release TRENTAL tablets for up to 60 weeks or immediate-release TRENTAL capsules for up to 24 weeks. Dosage ranges in the tablet studies were 400 mg bid to tid and in the capsule studies, 200–400 mg tid. The table summarizes the incidence (in percent) of adverse reactions considered drug related, as well as the numbers of patients who received extended-release TRENTAL tablets, immediate-release TRENTAL capsules, or the corresponding placebos. The incidence of adverse reactions was higher in the capsule studies (where dose related increases were seen in digestive and nervous system side effects) than in the tablet studies. Studies with the capsule include domestic experience, whereas studies with the extended-release tablets were conducted outside the U.S. The table indicates that in the tablet studies few patients discontinued because of adverse effects.
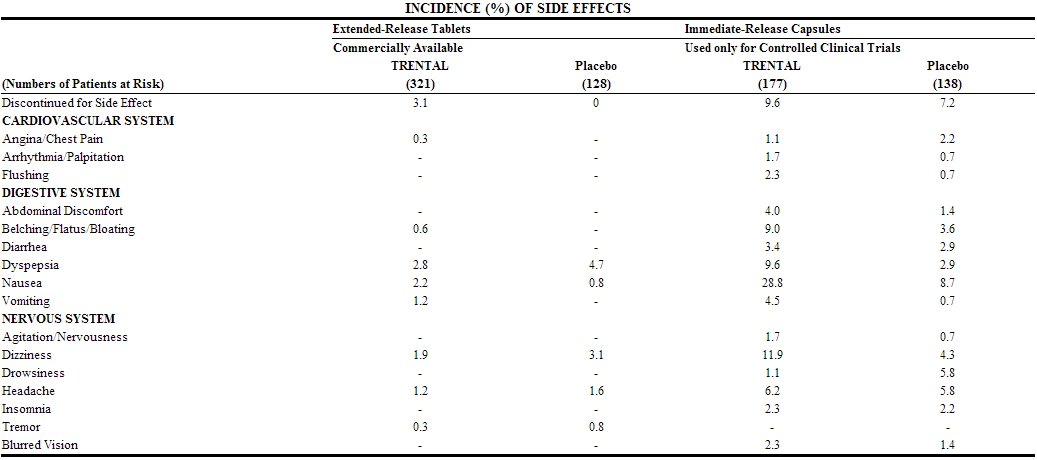
TRENTAL has been marketed in Europe and elsewhere since 1972. In addition to the above symptoms, the following have been reported spontaneously since marketing or occurred in other clinical trials with an incidence of less than 1%; the causal relationship was uncertain:
Cardiovascular - dyspnea, edema, hypotension.
Digestive - anorexia, cholecystitis, constipation, dry mouth/thirst.
Nervous - anxiety, confusion, depression, seizures, aseptic meningitis.
Respiratory - epistaxis, flu-like symptoms, laryngitis, nasal congestion.
Skin and Appendages - brittle fingernails, pruritus,rash, urticaria, angioedema.
Special Senses - blurred vision, conjunctivitis, earache, scotoma.
Miscellaneous - bad taste, excessive salivation, leukopenia, malaise, sore throat/swollen neck glands, weight change.
Postmarketing Experience
A few rare events have been reported spontaneously worldwide since marketing in 1972. Although they occurred under circumstances in which a causal relationship with pentoxifylline could not be established, they are listed to serve as information for physicians. Cardiovascular — angina, arrhythmia, tachycardia. Digestive — hepatitis, jaundice, cholestasis, increased liver enzymes; and Hemic and Lymphatic — decreased serum fibrinogen, pancytopenia, aplastic anemia, leukemia, purpura, thrombocytopenia. Immune system disorders — anaphylactic reaction, anaphylactoid reaction, anaphylactic shock.
Drug Interactions
Although a causal relationship has not been established, there have been reports of bleeding and/or prolonged prothrombin time in patients treated with TRENTAL with and without anticoagulants or platelet aggregation inhibitors. Patients on Warfarin should have more frequent monitoring of prothrombin times, while patients with other risk factors complicated by hemorrhage (e.g., recent surgery, peptic ulceration) should have periodic examinations for bleeding including hematocrit and/or hemoglobin. Concomitant administration of TRENTAL and theophylline-containing drugs leads to increased theophylline levels and theophylline toxicity in some individuals. Such patients should be closely monitored for signs of toxicity and have their theophylline dosage adjusted as necessary. TRENTAL has been used concurrently with beta blockers, digitalis, diuretics, antidiabetic agents, and antiarrhythmics, without observed problems. Small decreases in blood pressure have been observed in some patients treated with TRENTAL plus nifedipine or captopril; periodic systemic blood pressure monitoring is recommended for patients receiving concomitant antihypertensive therapy. If indicated, dosage of the antihypertensive agents should be reduced. Postmarketing cases of increased anticoagulant activity have been reported in patients concomitantly treated with pentoxifylline and vitamin K antagonists. Monitoring of anticoagulant activity in these patients is recommended when pentoxifylline is introduced or the dose is changed.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C
Teratogenicity studies have been performed in rats and rabbits using oral doses up to 576 and 264 mg/kg, respectively. On a weight basis, these doses are 24 and 11 times the maximum recommended human daily dose (MRHD); on a body-surface-area basis, they are 4.2 and 3.5 times the MRHD. No evidence of fetal malformation was observed. Increased resorption was seen in rats of the 576 mg/kg group. There are no adequate and well controlled studies in pregnant women. TRENTAL (pentoxifylline) should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Pentoxifylline in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Pentoxifylline during labor and delivery.
Nursing Mothers
Pentoxifylline and its metabolites are excreted in human milk. Because of the potential for tumorigenicity shown for pentoxifylline in rats, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
Clinical studies of TRENTAL did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. The active metabolite is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Gender
There is no FDA guidance on the use of Pentoxifylline with respect to specific gender populations.
Race
There is no FDA guidance on the use of Pentoxifylline with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Pentoxifylline in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Pentoxifylline in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Pentoxifylline in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Pentoxifylline in patients who are immunocompromised.
Administration and Monitoring
Administration
The usual dosage of TRENTAL in extended-release tablet form is one tablet (400 mg) three times a day with meals. While the effect of TRENTAL may be seen within 2 to 4 weeks, it is recommended that treatment be continued for at least 8 weeks. Efficacy has been demonstrated in double-blind clinical studies of 6 months duration. Digestive and central nervous system side effects are dose related. If patients develop these effects it is recommended that the dosage be lowered to one tablet twice a day (800 mg/day). If side effects persist at this lower dosage, the administration of TRENTAL should be discontinued.
Monitoring
- Patients on Warfarin should have more frequent monitoring of prothrombin times, while patients with other risk factors complicated by hemorrhage (e.g., recent surgery, peptic ulceration, cerebral and/or retinal bleeding) should have periodic examinations for bleeding including, hematocrit and/or hemoglobin.
- Concomitant administration of TRENTAL and theophylline-containing drugs leads to increased theophylline levels and theophylline toxicity in some individuals. Such patients should be closely monitored for signs of toxicity and have their theophylline dosage adjusted as necessary.
- Periodic systemic blood pressure monitoring is recommended for patients receiving concomitant antihypertensive therapy
- Monitoring of anticoagulant activity in these patients is recommended when pentoxifylline is introduced or the dose is changed
- Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
IV Compatibility
There is limited information regarding the compatibility of Pentoxifylline and IV administrations.
Overdosage
Overdosage with TRENTAL has been reported in pediatric patients and adults. Symptoms appear to be dose related. A report from a poison control center on 44 patients taking overdoses of enteric-coated pentoxifylline tablets noted that symptoms usually occurred 4–5 hours after ingestion and lasted about 12 hours. The highest amount ingested was 80 mg/kg; flushing, hypotension, convulsions, somnolence, loss of consciousness, fever, and agitation occurred. All patients recovered. In addition to symptomatic treatment and gastric lavage, special attention must be given to supporting respiration, maintaining systemic blood pressure, and controlling convulsions. Activated charcoal has been used to absorb pentoxifylline in patients who have overdosed.
Pharmacology
There is limited information regarding Pentoxifylline Pharmacology in the drug label.
Mechanism of Action
Pentoxifylline and its metabolites improve the flow properties of blood by decreasing its viscosity. In patients with chronic peripheral arterial disease, this increases blood flow to the affected microcirculation and enhances tissue oxygenation. The precise mode of action of pentoxifylline and the sequence of events leading to clinical improvement are still to be defined. Pentoxifylline administration has been shown to produce dose-related hemorrheologic effects, lowering blood viscosity, and improving erythrocyte flexibility. Leukocyte properties of hemorrheologic importance have been modified in animal and in vitro human studies. Pentoxifylline has been shown to increase leukocyte deformability and to inhibit neutrophil adhesion and activation. Tissue oxygen levels have been shown to be significantly increased by therapeutic doses of pentoxifylline in patients with peripheral arterial disease.
Structure
There is limited information regarding Pentoxifylline Structure in the drug label.
Pharmacodynamics
TRENTAL® (pentoxifylline) tablets for oral administration contain 400 mg of the active drug and the following inactive ingredients: FD&C Red No. 3, hypromellose USP, magnesium stearate NF, polyethylene glycol NF, povidone USP, talc USP, titanium dioxide USP, and hydroxyethyl cellulose USP in an extended-release formulation. TRENTAL is a tri-substituted xanthine derivative designated chemically as 1-(5-oxohexyl)-3,7-dimethylxanthine that, unlike theophylline, is a hemorrheologic agent, i.e. an agent that affects blood viscosity. Pentoxifylline is soluble in water and ethanol, and sparingly soluble in toluene. The CAS Registry Number is 6493-05-6. The chemical structure is:
thumb|400px|This image is provided by the National Library of Medicine.
Pharmacokinetics
After oral administration in aqueous solution pentoxifylline is almost completely absorbed. It undergoes a first-pass effect and the various metabolites appear in plasma very soon after dosing. Peak plasma levels of the parent compound and its metabolites are reached within 1 hour. The major metabolites are Metabolite l (1-[5-hydroxyhexyl]-3,7-dimethylxanthine) and Metabolite V (1-[3-carboxypropyl]-3,7-dimethylxanthine), and plasma levels of these metabolites are 5 and 8 times greater, respectively, than pentoxifylline. Following oral administration of aqueous solutions containing 100 to 400 mg of pentoxifylline, the pharmacokinetics of the parent compound and Metabolite l are dose-related and not proportional (non-linear), with half-life and area under the blood-level time curve (AUC) increasing with dose. The elimination kinetics of Metabolite V are not dose-dependent. The apparent plasma half-life of pentoxifylline varies from 0.4 to 0.8 hours and the apparent plasma half-lives of its metabolites vary from 1 to 1.6 hours. There is no evidence of accumulation or enzyme induction (Cytochrome P450) following multiple oral doses. Excretion is almost totally urinary; the main biotransformation product is Metabolite V. Essentially no parent drug is found in the urine. Despite large variations in plasma levels of parent compound and its metabolites, the urinary recovery of Metabolite V is consistent and shows dose proportionality. Less than 4% of the administered dose is recovered in feces. Food intake shortly before dosing delays absorption of an immediate-release dosage form but does not affect total absorption. The pharmacokinetics and metabolism of TRENTAL have not been studied in patients with renal and/or hepatic dysfunction. The pentoxifylline AUC was increased and elimination rate decreased in an older population (60–68 years, n=6) compared to younger individuals (22–30 years, n=6) (see PRECAUTIONS, Geriatric Use). After administration of the 400 mg extended-release TRENTAL tablet, plasma levels of the parent compound and its metabolites reach their maximum within 2 to 4 hours and remain constant over an extended period of time. Coadministration of TRENTAL tablets with meals resulted in an increase in mean Cmax and AUC by about 28% and 13% for pentoxifylline, respectively. Cmax for Metabolite 1 also increased by about 20%. The extended release of pentoxifylline from the tablet eliminates peaks and troughs in plasma levels for improved gastrointestinal tolerance.
Nonclinical Toxicology
Long-term studies of the carcinogenic potential of pentoxifylline were conducted in mice and rats by dietary administration of the drug at doses up to 450 mg/kg (approximately 19 times the maximum recommended human daily dose (MRHD) in both species when based on body weight; 1.5 times the MRHD in the mouse and 3.3 times the MRHD in the rat when based on body surface area). In mice, the drug was administered for 18 months, whereas in rats, the drug was administered for 18 months followed by an additional 6 months without drug exposure. In the rat study, there was a statistically significant increase in benign mammary fibroadenomas in females of the 450 mg/kg group. The relevance of this finding to human use is uncertain. Pentoxifylline was devoid of mutagenic activity in various strains of Salmonella (Ames test) and in cultured mammalian cells (unscheduled DNA synthesis test) when tested in the presence and absence of metabolic activation. It was also negative in the in vivo mouse micronucleus test.
Clinical Studies
There is limited information regarding Pentoxifylline Clinical Studies in the drug label.
How Supplied
TRENTAL (pentoxifylline) is available for oral administration as 400-mg pink film-coated oblong tablets imprinted Trental; supplied in bottles of 100 (NDC 0039-0078-10).
Storage
Store between 59 and 86° F (15 and 30° C). Dispense in well-closed, light-resistant containers.
Images
Drug Images
{{#ask: Page Name::Pentoxifylline |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Pentoxifylline |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Pentoxifylline Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Pentoxifylline interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Pentoxifylline Brand Names in the drug label.
Look-Alike Drug Names
TRENtal - TEGretol[27]
Drug Shortage Status
Price
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ De BK, Gangopadhyay S, Dutta D, Baksi SD, Pani A, Ghosh P (2009) Pentoxifylline versus prednisolone for severe alcoholic hepatitis: a randomized controlled trial. World J Gastroenterol 15 (13):1613-9. PMID: 19340904
- ↑ Louvet A, Diaz E, Dharancy S, Coevoet H, Texier F, Thévenot T et al. (2008) Early switch to pentoxifylline in patients with severe alcoholic hepatitis is inefficient in non-responders to corticosteroids. J Hepatol 48 (3):465-70. DOI:10.1016/j.jhep.2007.10.010 PMID: 18164508
- ↑ Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O (2000) Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology 119 (6):1637-48. PMID: 11113085
- ↑ Blume J, Rùhlmann KU, de la Haye R, Rettig K (1992) Treatment of chronic cerebrovascular disease in elderly patients with pentoxifylline. J Med 23 (6):417-32. PMID: 1293252
- ↑ (1996) European Pentoxifylline Multi-Infarct Dementia Study. Eur Neurol 36 (5):315-21. PMID: 8864715
- ↑ Parnetti L, Ciuffetti G, Mercuri M, Lupattelli G, Senin U (1986) The role of haemorheological factors in the ageing brain: long-term therapy with pentoxifylline ('Trental' 400) in elderly patients with initial mental deterioration. Pharmatherapeutica 4 (10):617-27. PMID: 3602014
- ↑ Herskovits E, Vazquez A, Famulari A, Smud R, Tamaroff L, Fraiman H et al. (1981) Randomised trial of pentoxifylline versus acetylsalicylic acid plus dipyridamole in preventing transient ischaemic attacks. Lancet 1 (8227):966-8. PMID: 6112386
- ↑ Sliwa K, Skudicky D, Candy G, Bergemann A, Hopley M, Sareli P (2002) The addition of pentoxifylline to conventional therapy improves outcome in patients with peripartum cardiomyopathy. Eur J Heart Fail 4 (3):305-9. PMID: 12034156
- ↑ Sliwa K, Skudicky D, Candy G, Wisenbaugh T, Sareli P (1998) Randomised investigation of effects of pentoxifylline on left-ventricular performance in idiopathic dilated cardiomyopathy. Lancet 351 (9109):1091-3. DOI:10.1016/S0140-6736(97)09338-0 PMID: 9660578
- ↑ Schwartz RW, Logan NM, Johnson PJ, Strodel WE, Fine JG, Kazmers A et al. (1989) Pentoxifylline increases extremity blood flow in diabetic atherosclerotic patients. Arch Surg 124 (4):434-7. PMID: 2649042
- ↑ Ghorbani A, Omidvar B, Beladi-Mousavi SS, Lak E, Vaziri S (2012) The effect of pentoxifylline on reduction of proteinuria among patients with type 2 diabetes under blockade of angiotensin system: a double blind and randomized clinical trial. Nefrologia 32 (6):790-6. DOI:10.3265/Nefrologia.pre2012.Jun.11242 PMID: 23169362
- ↑ Guerrero-Romero F, Rodríguez-Morán M, Paniagua-Sierra JR, García-Bulnes G, Salas-Ramírez M, Amato D (1995) Pentoxifylline reduces proteinuria in insulin-dependent and non insulin-dependent diabetic patients. Clin Nephrol 43 (2):116-21. PMID: 7736673
- ↑ Carr ME, Sanders K, Todd WM (1994) Pain relief and clinical improvement temporally related to the use of pentoxifylline in a patient with documented cholesterol emboli--a case report. Angiology 45 (1):65-9. PMID: 8285387
- ↑ Shen MR, Chiang PH, Yang RC, Hong CY, Chen SS (1991) Pentoxifylline stimulates human sperm motility both in vitro and after oral therapy. Br J Clin Pharmacol 31 (6):711-4. PMID: 1867966
- ↑ Safarinejad MR, Asgari MA, Hosseini SY, Dadkhah F (2010) A double-blind placebo-controlled study of the efficacy and safety of pentoxifylline in early chronic Peyronie's disease. BJU Int 106 (2):240-8. DOI:10.1111/j.1464-410X.2009.09041.x PMID: 19863517
- ↑ De Sanctis MT, Cesarone MR, Belcaro G, Incandela L, Steigerwalt R, Nicolaides AN et al. (2002) Treatment of retinal vein thrombosis with pentoxifylline: a controlled, randomized trial. Angiology 53 Suppl 1 ():S35-8. PMID: 11865834
- ↑ Incandela L, Cesarone MR, Belcaro G, Steigerwalt R, De Sanctis MT, Nicolaides AN et al. (2002) Treatment of vascular retinal disease with pentoxifylline: a controlled, randomized trial. Angiology 53 Suppl 1 ():S31-4. PMID: 11865833
- ↑ Jull AB, Arroll B, Parag V, Waters J (2012) Pentoxifylline for treating venous leg ulcers. Cochrane Database Syst Rev 12 ():CD001733. DOI:10.1002/14651858.CD001733.pub3 PMID: 23235582
- ↑ Radmilović A, Borić Z, Naumović T, Stamenković M, Muśikić P (1987) Shunt thrombosis prevention in hemodialysis patients--a double-blind, randomized study: pentoxifylline vs placebo. Angiology 38 (7):499-506. PMID: 3304027
- ↑ Lucas MA (1984) Prevention of post-operative thrombosis in peripheral arteriopathies. Pentoxifylline vs. conventional antiaggregants: a six-month randomized follow-up study. Angiology 35 (7):443-50. PMID: 6465619
- ↑ Young BA, Marsh CL, Alpers CE, Davis CL (1996) Cyclosporine-associated thrombotic microangiopathy/hemolytic uremic syndrome following kidney and kidney-pancreas transplantation. Am J Kidney Dis 28 (4):561-71. PMID: 8840947
- ↑ Incandela L, Cesarone MR, Belcaro G, De Sanctis MT, Nicolaides AN, Griffin M et al. (2002) Treatment of vascular inner ear disease with pentoxifylline: a 4-week, controlled, randomized trial. Angiology 53 Suppl 1 ():S19-22. PMID: 11865830
- ↑ Cesarone MR, Incandela L, Belcaro G, De Sanctis MT, Nicolaides AN, Griffin M et al. (2002) Treatment of vascular inner ear disease in vascular patients with pentoxifylline: a controlled, randomized trial. Angiology 53 Suppl 1 ():S23-6. PMID: 11865831
- ↑ Ely H, Bard JW (1988) Therapy of livedo vasculitis with pentoxifylline. Cutis 42 (5):448-53. PMID: 3197446
- ↑ Nürnberg W, Grabbe J, Czarnetzki BM (1994) Synergistic effects of pentoxifylline and dapsone in leucocytoclastic vasculitis. Lancet 343 (8895):491. PMID: 7906000
- ↑ Calderón MJ, Landa N, Aguirre A, Diaz-Perez JL (1993) Successful treatment of cutaneous PAN with pentoxifylline. Br J Dermatol 128 (6):706-8. PMID: 8101717
- ↑ "https://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Pentoxifylline |Pill Name=Pentoxifylline_400 mg_NDC 0093-5116.jpg |Drug Name=Pentoxifylline |Pill Ingred=hydroxyethyl cellulose (2000 mpa.s at 1%), isopropyl alcohol, magnesium stearate, talc|+sep=; |Pill Imprint=BVF;0117 |Pill Dosage=400 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=16.00 |Pill Scoring=1 |Pill Image= |Drug Author=Teva Pharmaceuticals USA Inc |NDC=0093-5116
}}
{{#subobject:
|Label Page=Pentoxifylline |Label Name=Pentoxifylline_label_01.jpg
}}
{{#subobject:
|Label Page=Pentoxifylline |Label Name=Pentoxifylline_panel_01.jpg
}}