Wild-type (senile) amyloidosis pathophysiology: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
|||
| Line 30: | Line 30: | ||
==Gross Pathology== | ==Gross Pathology== | ||
Cardiac amyloid deposits are most commonly seen in the [[myocardium]], but can also be seen in the [[atrium (heart)|atria]], [[pericardium]], [[endocardium]] and microvasculature. | |||
* On gross examination, the myocardium is thicker, firm and rubbery in consistency. More than half of myocardium involvement is common in the AL type of cardiac amyloidosis. | |||
* The size of the ventricular cavity remains unchanged, however filling of the ventricles is restricted (causing [[restrictive cardiomyopathy]]) because of stiffening of the ventricular wall as a result of deposition of amyloid material. | |||
* [[Pericardial effusion]] and valvular dysfunction is common from pericardial and endocardial involvement. [[Intracardiac thrombus]] formation is frequently seen and may result in fatal [[thromboembolism]].<ref name="pmid22583098">{{cite journal |author=Nakagawa M, Tojo K, Sekijima Y, Yamazaki KH, Ikeda S |title=Arterial thromboembolism in senile systemic amyloidosis: report of two cases |journal=[[Amyloid : the International Journal of Experimental and Clinical Investigation : the Official Journal of the International Society of Amyloidosis]] |volume=19 |issue=2 |pages=118–21 |year=2012 |month=June |pmid=22583098 |doi=10.3109/13506129.2012.685131 |url=}}</ref><ref name="pmid21893485">{{cite journal |author=Van de Veire NR, Dierick J, De Sutter J |title=Intracardiac emboli as first presentation of cardiac AL amyloidosis |journal=[[European Heart Journal]] |volume=33 |issue=7 |pages=818 |year=2012 |month=April |pmid=21893485 |pmc=3345559 |doi=10.1093/eurheartj/ehr330 |url=}}</ref><ref name="pmid17984380">{{cite journal |author=Feng D, Edwards WD, Oh JK, ''et al.'' |title=Intracardiac thrombosis and embolism in patients with cardiac amyloidosis |journal=[[Circulation]] |volume=116 |issue=21 |pages=2420–6 |year=2007 |month=November |pmid=17984380 |doi=10.1161/CIRCULATIONAHA.107.697763 |url=}}</ref> | |||
===Images=== | |||
[http://www.peir.net Images shown below are courtesy of Professor Peter Anderson and published with permission. © PEIR, University of Alabama at Birmingham, Department of Pathology] | [http://www.peir.net Images shown below are courtesy of Professor Peter Anderson and published with permission. © PEIR, University of Alabama at Birmingham, Department of Pathology] | ||
{| align="left" | {| align="left" | ||
Revision as of 16:36, 17 December 2019
|
Wild-type (senile) amyloidosis Microchapters |
|
Differentiating Wild-type (senile) amyloidosis from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Wild-type (senile) amyloidosis pathophysiology On the Web |
|
American Roentgen Ray Society Images of Wild-type (senile) amyloidosis pathophysiology |
|
Risk calculators and risk factors for Wild-type (senile) amyloidosis pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]Associate Editor(s)-in-Chief: Sabawoon Mirwais, M.B.B.S, M.D.[2]
Overview
Pathophysiology
- Amyloid is an abnormal insoluble extracellular protein that deposits in the different tissues and causes organic dysfunction and a wide variety of clinical syndromes.[1]
- These abnormal amyloids are derived from misfolding and aggregation of normally soluble proteins.
- Amyloid deposition can disrupt tissue structure of involved organ and consequently leads to organ failure.[2]
Systemic Amyloidosis
- In systemic amyloidosis, amyloid gradually accumulates and amyloid deposition is widespread in the viscera, blood vessel walls, and different connective tissues.[3][4]
Pathogenesis
- Wild-type (senile) amyloidosis is a type of systemic amyloidosis as transthyretin (TTR) deposits can be found throughout the body.
- The culprit protein responsible for the disease is TTR and it is deposited in the non-mutated form, hence the name "Wild-type".
- TTR results in pathologies due to misfolding, breaking apart, and deposition of the amyloid fibrils in healthy tissue.
- The normal TTR protein, compared with the mutated form, is less likely to get deposited and cause pathology.
- This is believed to be the reason as to why this condition almost always affects the elderly (65 years of age or older).
- The condition mainly affects the heart. However, other organ systems, such as the nervous and musculoskeletal systems, can also be involved.
Genetics
- There are no genes implicated in the causality of wild-type (senile) amyloidosis.
Associated Conditions
- Aging is very strongly associated with wild-type (senile) amyloidosis.
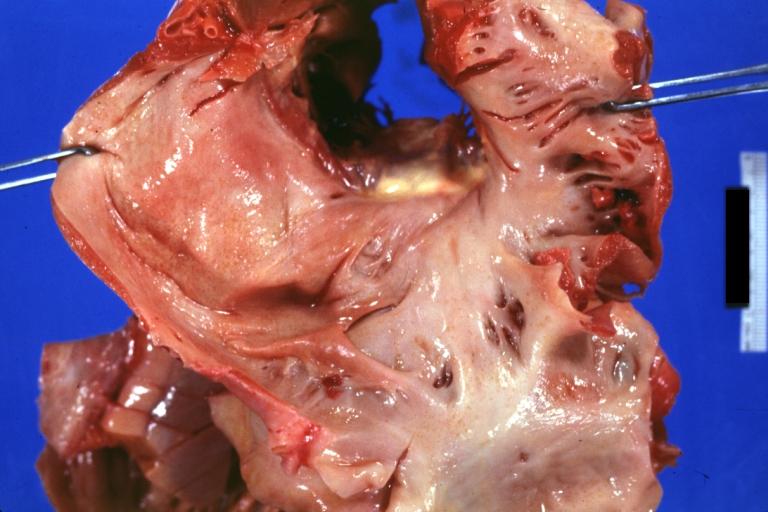
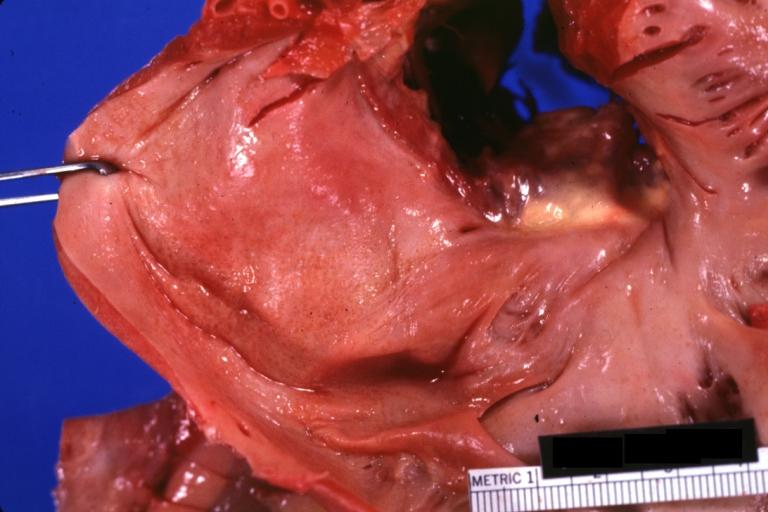
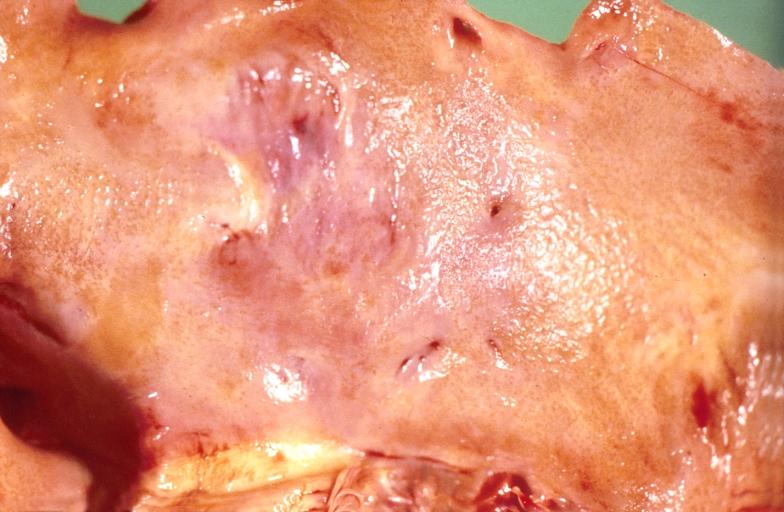
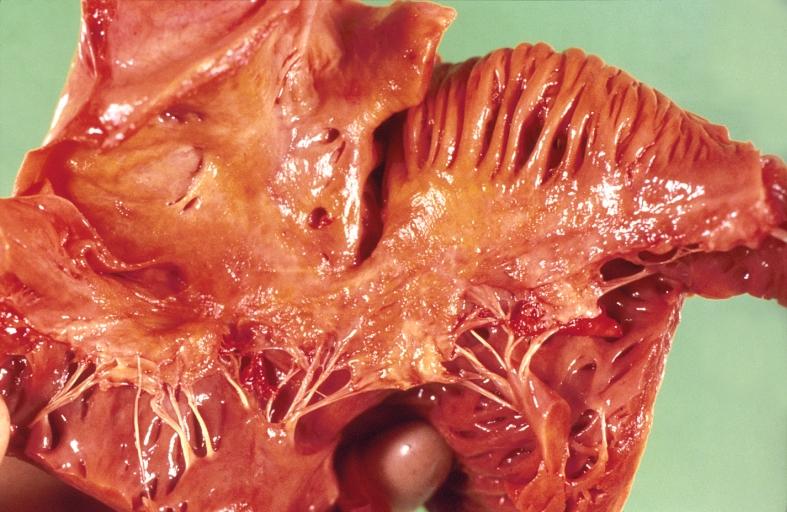
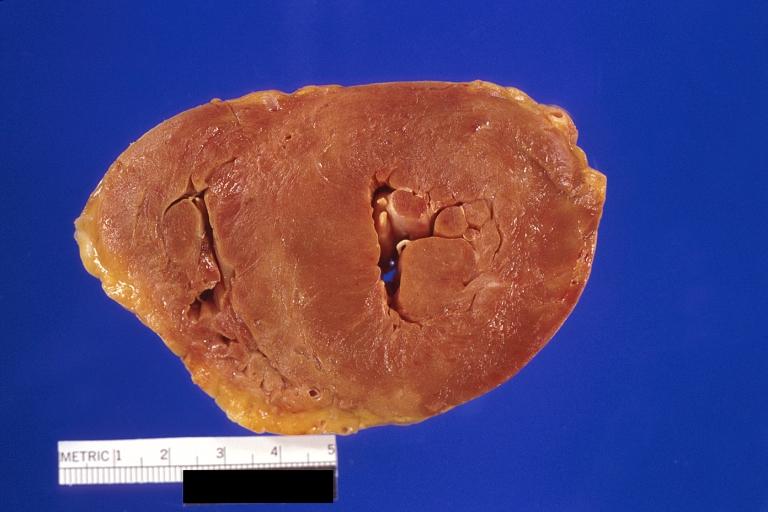
Gross Pathology
Cardiac amyloid deposits are most commonly seen in the myocardium, but can also be seen in the atria, pericardium, endocardium and microvasculature.
- On gross examination, the myocardium is thicker, firm and rubbery in consistency. More than half of myocardium involvement is common in the AL type of cardiac amyloidosis.
- The size of the ventricular cavity remains unchanged, however filling of the ventricles is restricted (causing restrictive cardiomyopathy) because of stiffening of the ventricular wall as a result of deposition of amyloid material.
- Pericardial effusion and valvular dysfunction is common from pericardial and endocardial involvement. Intracardiac thrombus formation is frequently seen and may result in fatal thromboembolism.[5][6][7]
Images
 |
 |
 |
 |
 |
References
- ↑ Wechalekar AD, Gillmore JD, Hawkins PN (June 2016). "Systemic amyloidosis". Lancet. 387 (10038): 2641–2654. doi:10.1016/S0140-6736(15)01274-X. PMID 26719234.
- ↑ Wechalekar AD, Gillmore JD, Hawkins PN (June 2016). "Systemic amyloidosis". Lancet. 387 (10038): 2641–2654. doi:10.1016/S0140-6736(15)01274-X. PMID 26719234.
- ↑ Baker KR, Rice L (2012). "The amyloidoses: clinical features, diagnosis and treatment". Methodist Debakey Cardiovasc J. 8 (3): 3–7. PMC 3487569. PMID 23227278.
- ↑ Pepys MB (2006). "Amyloidosis". Annu. Rev. Med. 57: 223–41. doi:10.1146/annurev.med.57.121304.131243. PMID 16409147.
- ↑ Nakagawa M, Tojo K, Sekijima Y, Yamazaki KH, Ikeda S (2012). "Arterial thromboembolism in senile systemic amyloidosis: report of two cases". Amyloid : the International Journal of Experimental and Clinical Investigation : the Official Journal of the International Society of Amyloidosis. 19 (2): 118–21. doi:10.3109/13506129.2012.685131. PMID 22583098. Unknown parameter
|month=ignored (help) - ↑ Van de Veire NR, Dierick J, De Sutter J (2012). "Intracardiac emboli as first presentation of cardiac AL amyloidosis". European Heart Journal. 33 (7): 818. doi:10.1093/eurheartj/ehr330. PMC 3345559. PMID 21893485. Unknown parameter
|month=ignored (help) - ↑ Feng D, Edwards WD, Oh JK; et al. (2007). "Intracardiac thrombosis and embolism in patients with cardiac amyloidosis". Circulation. 116 (21): 2420–6. doi:10.1161/CIRCULATIONAHA.107.697763. PMID 17984380. Unknown parameter
|month=ignored (help)