Doripenem description: Difference between revisions
Gerald Chi (talk | contribs) mNo edit summary |
|||
| (One intermediate revision by the same user not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{Doripenem}} | {{Doripenem}} | ||
{{CMG}} | {{CMG}}; {{AE}} {{SS}} | ||
==Description== | ==Description== | ||
DORIBAX®, doripenem monohydrate for injection vials contain 500 mg of doripenem on an anhydrous basis, a white to slightly-yellowish off-white sterile crystalline powder. All references to doripenem activity are expressed in terms of the active doripenem moiety. The powder is constituted for intravenous infusion. The pH of the infusion solution is between 4.5 and 5.5. | DORIBAX®, doripenem monohydrate for injection vials contain 500 mg of doripenem on an anhydrous basis, a white to slightly-yellowish off-white sterile crystalline powder. All references to doripenem activity are expressed in terms of the active doripenem moiety. The powder is constituted for intravenous infusion. The pH of the infusion solution is between 4.5 and 5.5. | ||
| Line 11: | Line 12: | ||
Its molecular weight is 438.52, and its chemical structure is:<ref>{{Cite web | last = | first = |title =http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022106s014lbl.pdf | url =http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022106s014lbl.pdf | publisher =|date = | accessdate = }}</ref> | Its molecular weight is 438.52, and its chemical structure is:<ref>{{Cite web | last = | first = |title =http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022106s014lbl.pdf | url =http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022106s014lbl.pdf | publisher =|date = | accessdate = }}</ref> | ||
{| | {| | ||
|- | |- | ||
Latest revision as of 22:08, 5 January 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Description
DORIBAX®, doripenem monohydrate for injection vials contain 500 mg of doripenem on an anhydrous basis, a white to slightly-yellowish off-white sterile crystalline powder. All references to doripenem activity are expressed in terms of the active doripenem moiety. The powder is constituted for intravenous infusion. The pH of the infusion solution is between 4.5 and 5.5.
DORIBAX® is not formulated with any inactive ingredients.
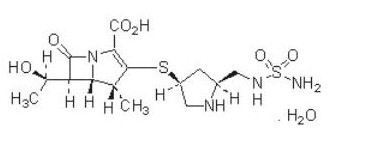
DORIBAX® (doripenem monohydrate) is a synthetic broad-spectrum carbapenem antibiotic structurally related to beta-lactam antibiotics. The chemical name for doripenem monohydrate is (4R,5S,6S)-3-[((3S,5S)-5-[[(aminosulfonyl)amino]methyl]-3-pyrrolidinyl)thio]-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid monohydrate.
Its molecular weight is 438.52, and its chemical structure is:[1]
 |
References
- ↑ "http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022106s014lbl.pdf" (PDF). External link in
|title=(help)
Adapted from the FDA Package Insert.