Fosamprenavir dosage and administration: Difference between revisions
Gerald Chi (talk | contribs) mNo edit summary |
Gerald Chi (talk | contribs) m (→Adults) |
||
| (2 intermediate revisions by the same user not shown) | |||
| Line 39: | Line 39: | ||
'''(Table 1)''' | '''(Table 1)''' | ||
Twice-Daily Dosage Regimens by Weight for | Twice-Daily Dosage Regimens by Weight for [[protease inhibitor]]-Naive Pediatric Patients (Greater Than or Equal to 4 Weeks of Age) and for [[protease inhibitor]]-Experienced Pediatric Patients (Greater Than or Equal to 6 Months of Age) Using LEXIVA Oral Suspension With Concurrent Ritonavir | ||
{| | {| | ||
| Line 45: | Line 45: | ||
|} | |} | ||
Alternatively, protease inhibitor-naive children aged 2 years and older can be administered LEXIVA (without ritonavir) 30 mg per kg twice daily. | Alternatively, [[protease inhibitor]]-naive children aged 2 years and older can be administered LEXIVA (without ritonavir) 30 mg per kg twice daily. | ||
LEXIVA should only be administered to infants born at 38 weeks gestation or greater and who have attained a post-natal age of 28 days. | LEXIVA should only be administered to infants born at 38 weeks gestation or greater and who have attained a post-natal age of 28 days. | ||
| Line 53: | Line 53: | ||
* Do not support once-daily dosing of LEXIVA alone or in combination with ritonavir. | * Do not support once-daily dosing of LEXIVA alone or in combination with ritonavir. | ||
* Do not support administration of LEXIVA alone or in combination with ritonavir for protease | * Do not support administration of LEXIVA alone or in combination with ritonavir for [[protease inhibitor]]‑experienced children younger than 6 months. | ||
* Do not support twice-daily dosing of LEXIVA without ritonavir in pediatric patients younger than 2 years . | * Do not support twice-daily dosing of LEXIVA without ritonavir in pediatric patients younger than 2 years . | ||
| Line 65: | Line 65: | ||
===Patients With Hepatic Impairment=== | ===Patients With Hepatic Impairment=== | ||
'''Mild Hepatic Impairment''' (Child-Pugh Score Ranging From 5 to 6): LEXIVA should be used with caution at a reduced dosage of 700 mg twice daily without ritonavir (therapy-naive) or 700 mg twice daily plus ritonavir 100 mg once daily (therapy-naive or protease inhibitor-experienced). | '''Mild Hepatic Impairment''' (Child-Pugh Score Ranging From 5 to 6): LEXIVA should be used with caution at a reduced dosage of 700 mg twice daily without ritonavir (therapy-naive) or 700 mg twice daily plus ritonavir 100 mg once daily (therapy-naive or [[protease inhibitor]]-experienced). | ||
'''Moderate Hepatic Impairment''' (Child-Pugh Score Ranging From 7 to 9): LEXIVA should be used with caution at a reduced dosage of 700 mg twice daily without ritonavir (therapy-naive), or 450 mg twice daily plus ritonavir 100 mg once daily (therapy-naive or protease inhibitor-experienced). | '''Moderate Hepatic Impairment''' (Child-Pugh Score Ranging From 7 to 9): LEXIVA should be used with caution at a reduced dosage of 700 mg twice daily without ritonavir (therapy-naive), or 450 mg twice daily plus ritonavir 100 mg once daily (therapy-naive or [[protease inhibitor]]-experienced). | ||
'''Severe Hepatic Impairment''' (Child-Pugh Score Ranging From 10 to 15): LEXIVA should be used with caution at a reduced dosage of 350 mg twice daily without ritonavir (therapy-naive) or 300 mg twice daily plus ritonavir 100 mg once daily (therapy-naive or protease inhibitor-experienced). | '''Severe Hepatic Impairment''' (Child-Pugh Score Ranging From 10 to 15): LEXIVA should be used with caution at a reduced dosage of 350 mg twice daily without ritonavir (therapy-naive) or 300 mg twice daily plus ritonavir 100 mg once daily (therapy-naive or [[protease inhibitor]]-experienced). | ||
There are no data to support dosing recommendations for pediatric patients with hepatic impairment.<ref>{{Cite web | last = | first = | title = LEXIVA (FOSAMPRENAVIR CALCIUM) TABLET, FILM COATED LEXIVA (FOSAMPRENAVIR CALCIUM) SUSPENSION [VIIV HEALTHCARE COMPANY] | url = http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=24feb9be-32a6-45fd-a896-f3e202edd8a9 | publisher = | date = | accessdate = }}</ref> | There are no data to support dosing recommendations for pediatric patients with hepatic impairment.<ref>{{Cite web | last = | first = | title = LEXIVA (FOSAMPRENAVIR CALCIUM) TABLET, FILM COATED LEXIVA (FOSAMPRENAVIR CALCIUM) SUSPENSION [VIIV HEALTHCARE COMPANY] | url = http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=24feb9be-32a6-45fd-a896-f3e202edd8a9 | publisher = | date = | accessdate = }}</ref> | ||
==References== | ==References== | ||
Latest revision as of 19:24, 5 January 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ahmed Zaghw, M.D. [2]
Dosage and Administration
LEXIVA Tablets may be taken with or without food.
Adults should take LEXIVA Oral Suspension without food. Pediatric patients should take LEXIVA Oral Suspension with food. If emesis occurs within 30 minutes after dosing, re-dosing of LEXIVA Oral Suspension should occur.
Higher-than-approved dose combinations of LEXIVA plus ritonavir are not recommended due to an increased risk of transaminase elevations.
When LEXIVA is used in combination with ritonavir, prescribers should consult the full prescribing information for ritonavir.
Adults
Therapy-Naive Adults
LEXIVA 1,400 mg twice daily (without ritonavir).
LEXIVA 1,400 mg once daily plus ritonavir 200 mg once daily.
LEXIVA 1,400 mg once daily plus ritonavir 100 mg once daily.
LEXIVA 1,400 mg once daily plus ritonavir 100 mg once daily is supported by pharmacokinetic data .
LEXIVA 700 mg twice daily plus ritonavir 100 mg twice daily.
LEXIVA 700 mg twice daily plus 100 mg ritonavir twice daily is supported by pharmacokinetic and safety data.
Protease Inhibitor-Experienced Adults
LEXIVA 700 mg twice daily plus ritonavir 100 mg twice daily.
Pediatric Patients (Aged at Least 4 Weeks to 18 Years)
The recommended dosage of LEXIVA in patients aged at least 4 weeks to 18 years should be calculated based on body weight (kg) and should not exceed the recommended adult dose (Table 1).
(Table 1)
Twice-Daily Dosage Regimens by Weight for protease inhibitor-Naive Pediatric Patients (Greater Than or Equal to 4 Weeks of Age) and for protease inhibitor-Experienced Pediatric Patients (Greater Than or Equal to 6 Months of Age) Using LEXIVA Oral Suspension With Concurrent Ritonavir
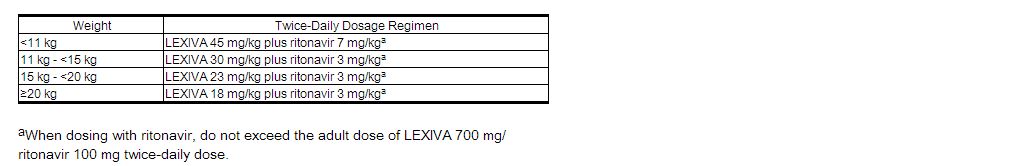 |
Alternatively, protease inhibitor-naive children aged 2 years and older can be administered LEXIVA (without ritonavir) 30 mg per kg twice daily.
LEXIVA should only be administered to infants born at 38 weeks gestation or greater and who have attained a post-natal age of 28 days.
For pediatric patients, pharmacokinetic and clinical data:
- Do not support once-daily dosing of LEXIVA alone or in combination with ritonavir.
- Do not support administration of LEXIVA alone or in combination with ritonavir for protease inhibitor‑experienced children younger than 6 months.
- Do not support twice-daily dosing of LEXIVA without ritonavir in pediatric patients younger than 2 years .
Other Dosing Considerations:
- When administered without ritonavir, the adult regimen of LEXIVA Tablets 1,400 mg twice daily may be used for pediatric patients weighing at least 47 kg.
- When administered in combination with ritonavir, LEXIVA Tablets may be used for pediatric patients weighing at least 39 kg; ritonavir capsules may be used for pediatric patients weighing at least 33 kg.
Patients With Hepatic Impairment
Mild Hepatic Impairment (Child-Pugh Score Ranging From 5 to 6): LEXIVA should be used with caution at a reduced dosage of 700 mg twice daily without ritonavir (therapy-naive) or 700 mg twice daily plus ritonavir 100 mg once daily (therapy-naive or protease inhibitor-experienced).
Moderate Hepatic Impairment (Child-Pugh Score Ranging From 7 to 9): LEXIVA should be used with caution at a reduced dosage of 700 mg twice daily without ritonavir (therapy-naive), or 450 mg twice daily plus ritonavir 100 mg once daily (therapy-naive or protease inhibitor-experienced).
Severe Hepatic Impairment (Child-Pugh Score Ranging From 10 to 15): LEXIVA should be used with caution at a reduced dosage of 350 mg twice daily without ritonavir (therapy-naive) or 300 mg twice daily plus ritonavir 100 mg once daily (therapy-naive or protease inhibitor-experienced).
There are no data to support dosing recommendations for pediatric patients with hepatic impairment.[1]
References
Adapted from the FDA Package Insert.