Fosamprenavir clinical studies: Difference between revisions
Ahmed Zaghw (talk | contribs) (Created page with "__NOTOC__ {{Fosamprenavir}} {{CMG}}; {{AE}} {{AZ}} <ref>{{Cite web | last = | first = | title = LEXIVA (FOSAMPRENAVIR CALCIUM) TABLET, FILM COATED LEXIVA (FOSAMPRENAVI...") |
Gerald Chi (talk | contribs) mNo edit summary |
||
| (One intermediate revision by one other user not shown) | |||
| Line 3: | Line 3: | ||
{{CMG}}; {{AE}} {{AZ}} | {{CMG}}; {{AE}} {{AZ}} | ||
==Clinical Studies== | |||
===Therapy-Naive Adult Trials=== | |||
* '''APV30001''' | |||
A randomized, open-label trial evaluated treatment with LEXIVA Tablets (1,400 mg twice daily) versus nelfinavir (1,250 mg twice daily) in 249 antiretroviral treatment-naive subjects. Both groups of subjects also received abacavir (300 mg twice daily) and lamivudine (150 mg twice daily). | |||
The mean age of the subjects in this trial was 37 years (range: 17 to 70 years); 69% of the subjects were male, 20% were CDC Class C (AIDS), 24% were Caucasian, 32% were black, and 44% were Hispanic. At baseline, the median CD4+ cell count was 212 cells per mm3 (range: 2 to 1,136 cells per mm3; 18% of subjects had a CD4+ cell count of less than 50 cells per mm3 and 30% were in the range of 50 to less than 200 cells per mm3). Baseline median HIV-1 RNA was 4.83 log10 copies per mL (range: 1.69 to 7.41 log10 copies per mL; 45% of subjects had greater than 100,000 copies per mL). | |||
The outcomes of randomized treatment are provided in Table 15. | |||
{| | |||
|[[File:15.JPG|600px|thumb]] | |||
|} | |||
Treatment response by viral load strata is shown in Table 16. | |||
{| | |||
|[[File:16.JPG|600px|thumb]] | |||
|} | |||
Through 48 weeks of therapy, the median increases from baseline in CD4+ cell counts were 201 cells per mm3 in the group receiving LEXIVA and 216 cells per mm3 in the nelfinavir group. | |||
* '''APV30002''' | |||
A randomized, open-label trial evaluated treatment with LEXIVA Tablets (1,400 mg once daily) plus ritonavir (200 mg once daily) versus nelfinavir (1,250 mg twice daily) in 649 treatment-naive subjects. Both treatment groups also received abacavir (300 mg twice daily) and lamivudine (150 mg twice daily). | |||
The mean age of the subjects in this trial was 37 years (range: 18 to 69 years); 73% of the subjects were male, 22% were CDC Class C, 53% were Caucasian, 36% were black, and 8% were Hispanic. At baseline, the median CD4+ cell count was 170 cells per mm3 (range: 1 to 1,055 cells per mm3; 20% of subjects had a CD4+ cell count of less than 50 cells per mm3 and 35% were in the range of 50 to less than 200 cells per mm3). Baseline median HIV-1 RNA was 4.81 log10 copies per mL (range: 2.65 to 7.29 log10 copies per mL; 43% of subjects had greater than 100,000 copies per mL). | |||
The outcomes of randomized treatment are provided in Table 17. | |||
{| | |||
|[[File:17.JPG|600px|thumb]] | |||
|} | |||
Treatment response by viral load strata is shown in Table 18. | |||
{| | |||
|[[File:18.JPG|600px|thumb]] | |||
|} | |||
===Protease Inhibitor-Experienced Adult Trials=== | |||
* '''APV30003''' | |||
A randomized, open-label, multicenter trial evaluated 2 different regimens of LEXIVA plus ritonavir (LEXIVA Tablets 700 mg twice daily plus ritonavir 100 mg twice daily or LEXIVA Tablets 1,400 mg once daily plus ritonavir 200 mg once daily) versus lopinavir/ritonavir (400 mg/100 mg twice daily) in 315 subjects who had experienced virologic failure to 1 or 2 prior protease inhibitor-containing regimens. | |||
The mean age of the subjects in this trial was 42 years (range: 24 to 72 years); 85% were male, 33% were CDC Class C, 67% were Caucasian, 24% were black, and 9% were Hispanic. The median CD4+ cell count at baseline was 263 cells per mm3 (range: 2 to 1,171 cells per mm3). Baseline median plasma HIV-1 RNA level was 4.14 log10 copies per mL (range: 1.69 to 6.41 log10 copies per mL). | |||
The median durations of prior exposure to NRTIs were 257 weeks for subjects receiving LEXIVA/ritonavir twice daily (79% had greater than or equal to 3 prior NRTIs) and 210 weeks for subjects receiving lopinavir/ritonavir (64% had greater than or equal to 3 prior NRTIs). The median durations of prior exposure to protease inhibitors were 149 weeks for subjects receiving LEXIVA/ritonavir twice daily (49% received greater than or equal to 2 prior protease inhibitors) and 130 weeks for subjects receiving lopinavir/ritonavir (40% received greater than or equal to 2 prior protease inhibitors). | |||
The time-averaged changes in plasma HIV-1 RNA from baseline (AAUCMB) at 48 weeks (the endpoint on which the trial was powered) were -1.4 log10 copies per mL for twice-daily LEXIVA/ritonavir and -1.67 log10 copies per mL for the lopinavir/ritonavir group. | |||
The proportions of subjects who achieved and maintained confirmed HIV-1 RNA less than 400 copies per mL (secondary efficacy endpoint) were 58% with twice-daily LEXIVA/ritonavir and 61% with lopinavir/ritonavir (95% CI for the difference: -16.6, 10.1). The proportions of subjects with HIV-1 RNA less than 50 copies per mL with twice-daily LEXIVA/ritonavir and with lopinavir/ritonavir were 46% and 50%, respectively (95% CI for the difference: -18.3, 8.9). The proportions of subjects who were virologic failures were 29% with twice-daily LEXIVA/ritonavir and 27% with lopinavir/ritonavir. | |||
The frequency of discontinuations due to adverse events and other reasons, and deaths were similar between treatment arms. | |||
Through 48 weeks of therapy, the median increases from baseline in CD4+ cell counts were 81 cells per mm3 with twice-daily LEXIVA/ritonavir and 91 cells per mm3 with lopinavir/ritonavir. | |||
This trial was not large enough to reach a definitive conclusion that LEXIVA/ritonavir and lopinavir/ritonavir are clinically equivalent. | |||
Once-daily administration of LEXIVA plus ritonavir is not recommended for protease inhibitor-experienced patients. Through Week 48, 50% and 37% of subjects receiving LEXIVA 1,400 mg plus ritonavir 200 mg once daily had plasma HIV-1 RNA less than 400 copies per mL and less than 50 copies per mL, respectively. | |||
===Pediatric Trials=== | |||
Three open-label trials in pediatric subjects aged at least 4 weeks to 18 years were conducted. In one trial (APV29005), twice-daily dosing regimens (LEXIVA with or without ritonavir) were evaluated in combination with other antiretroviral agents in pediatric subjects aged 2 to 18 years. In a second trial (APV20002), twice-daily dosing regimens (LEXIVA with ritonavir) were evaluated in combination with other antiretroviral agents in pediatric subjects aged at least 4 weeks to less than 2 years. A third trial (APV20003) evaluated once-daily dosing of LEXIVA with ritonavir; the pharmacokinetic data from this trial did not support a once-daily dosing regimen in any pediatric patient population. | |||
* '''APV29005 LEXIVA''' | |||
Twenty (18 therapy-naive and 2 therapy-experienced) pediatric subjects received LEXIVA Oral Suspension without ritonavir twice daily. At Week 24, 65% (13/20) achieved HIV-1 RNA less than 400 copies per mL, and the median increase from baseline in CD4+ cell count was 350 cells per mm3. | |||
LEXIVA plus Ritonavir: Forty-nine protease inhibitor-naive and 40 protease inhibitor-experienced pediatric subjects received LEXIVA Oral Suspension or Tablets with ritonavir twice daily. At Week 24, 71% of protease inhibitor-naive (35/49) and 55% of protease inhibitor-experienced (22/40) subjects achieved HIV-1 RNA less than 400 copies per mL; median increases from baseline in CD4+ cell counts were 184 cells per mm3 and 150 cells per mm3 in protease inhibitor-naive and experienced subjects, respectively. | |||
* '''APV20002''' | |||
Fifty-four pediatric subjects (49 protease inhibitor-naive and 5 protease inhibitor-experienced) received LEXIVA Oral Suspension with ritonavir twice daily. At Week 24, 72% of subjects achieved HIV-1 RNA less than 400 copies per mL. The median increases from baseline in CD4+ cell counts were 400 cells per mm3 in subjects aged at least 4 weeks to less than 6 months and 278 cells per mm3 in subjects aged 6 months to 2 years.<ref>{{Cite web | last = | first = | title = LEXIVA (FOSAMPRENAVIR CALCIUM) TABLET, FILM COATED LEXIVA (FOSAMPRENAVIR CALCIUM) SUSPENSION [VIIV HEALTHCARE COMPANY] | url = http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=24feb9be-32a6-45fd-a896-f3e202edd8a9 | publisher = | date = | accessdate = }}</ref> | |||
==References== | ==References== | ||
Latest revision as of 19:19, 5 January 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ahmed Zaghw, M.D. [2]
Clinical Studies
Therapy-Naive Adult Trials
- APV30001
A randomized, open-label trial evaluated treatment with LEXIVA Tablets (1,400 mg twice daily) versus nelfinavir (1,250 mg twice daily) in 249 antiretroviral treatment-naive subjects. Both groups of subjects also received abacavir (300 mg twice daily) and lamivudine (150 mg twice daily).
The mean age of the subjects in this trial was 37 years (range: 17 to 70 years); 69% of the subjects were male, 20% were CDC Class C (AIDS), 24% were Caucasian, 32% were black, and 44% were Hispanic. At baseline, the median CD4+ cell count was 212 cells per mm3 (range: 2 to 1,136 cells per mm3; 18% of subjects had a CD4+ cell count of less than 50 cells per mm3 and 30% were in the range of 50 to less than 200 cells per mm3). Baseline median HIV-1 RNA was 4.83 log10 copies per mL (range: 1.69 to 7.41 log10 copies per mL; 45% of subjects had greater than 100,000 copies per mL).
The outcomes of randomized treatment are provided in Table 15.
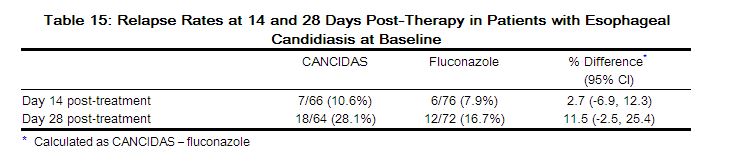 |
Treatment response by viral load strata is shown in Table 16.
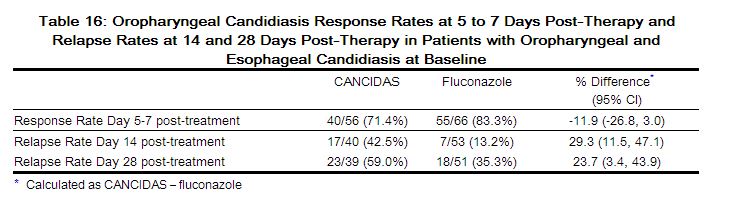 |
Through 48 weeks of therapy, the median increases from baseline in CD4+ cell counts were 201 cells per mm3 in the group receiving LEXIVA and 216 cells per mm3 in the nelfinavir group.
- APV30002
A randomized, open-label trial evaluated treatment with LEXIVA Tablets (1,400 mg once daily) plus ritonavir (200 mg once daily) versus nelfinavir (1,250 mg twice daily) in 649 treatment-naive subjects. Both treatment groups also received abacavir (300 mg twice daily) and lamivudine (150 mg twice daily).
The mean age of the subjects in this trial was 37 years (range: 18 to 69 years); 73% of the subjects were male, 22% were CDC Class C, 53% were Caucasian, 36% were black, and 8% were Hispanic. At baseline, the median CD4+ cell count was 170 cells per mm3 (range: 1 to 1,055 cells per mm3; 20% of subjects had a CD4+ cell count of less than 50 cells per mm3 and 35% were in the range of 50 to less than 200 cells per mm3). Baseline median HIV-1 RNA was 4.81 log10 copies per mL (range: 2.65 to 7.29 log10 copies per mL; 43% of subjects had greater than 100,000 copies per mL).
The outcomes of randomized treatment are provided in Table 17.
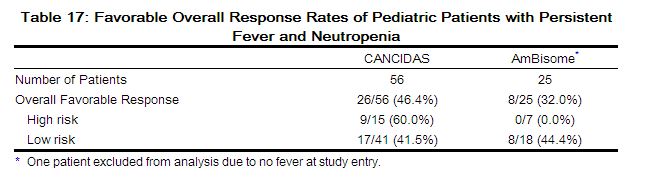 |
Treatment response by viral load strata is shown in Table 18.
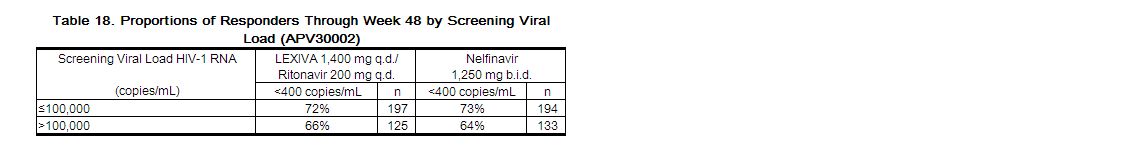 |
Protease Inhibitor-Experienced Adult Trials
- APV30003
A randomized, open-label, multicenter trial evaluated 2 different regimens of LEXIVA plus ritonavir (LEXIVA Tablets 700 mg twice daily plus ritonavir 100 mg twice daily or LEXIVA Tablets 1,400 mg once daily plus ritonavir 200 mg once daily) versus lopinavir/ritonavir (400 mg/100 mg twice daily) in 315 subjects who had experienced virologic failure to 1 or 2 prior protease inhibitor-containing regimens.
The mean age of the subjects in this trial was 42 years (range: 24 to 72 years); 85% were male, 33% were CDC Class C, 67% were Caucasian, 24% were black, and 9% were Hispanic. The median CD4+ cell count at baseline was 263 cells per mm3 (range: 2 to 1,171 cells per mm3). Baseline median plasma HIV-1 RNA level was 4.14 log10 copies per mL (range: 1.69 to 6.41 log10 copies per mL).
The median durations of prior exposure to NRTIs were 257 weeks for subjects receiving LEXIVA/ritonavir twice daily (79% had greater than or equal to 3 prior NRTIs) and 210 weeks for subjects receiving lopinavir/ritonavir (64% had greater than or equal to 3 prior NRTIs). The median durations of prior exposure to protease inhibitors were 149 weeks for subjects receiving LEXIVA/ritonavir twice daily (49% received greater than or equal to 2 prior protease inhibitors) and 130 weeks for subjects receiving lopinavir/ritonavir (40% received greater than or equal to 2 prior protease inhibitors).
The time-averaged changes in plasma HIV-1 RNA from baseline (AAUCMB) at 48 weeks (the endpoint on which the trial was powered) were -1.4 log10 copies per mL for twice-daily LEXIVA/ritonavir and -1.67 log10 copies per mL for the lopinavir/ritonavir group.
The proportions of subjects who achieved and maintained confirmed HIV-1 RNA less than 400 copies per mL (secondary efficacy endpoint) were 58% with twice-daily LEXIVA/ritonavir and 61% with lopinavir/ritonavir (95% CI for the difference: -16.6, 10.1). The proportions of subjects with HIV-1 RNA less than 50 copies per mL with twice-daily LEXIVA/ritonavir and with lopinavir/ritonavir were 46% and 50%, respectively (95% CI for the difference: -18.3, 8.9). The proportions of subjects who were virologic failures were 29% with twice-daily LEXIVA/ritonavir and 27% with lopinavir/ritonavir.
The frequency of discontinuations due to adverse events and other reasons, and deaths were similar between treatment arms.
Through 48 weeks of therapy, the median increases from baseline in CD4+ cell counts were 81 cells per mm3 with twice-daily LEXIVA/ritonavir and 91 cells per mm3 with lopinavir/ritonavir.
This trial was not large enough to reach a definitive conclusion that LEXIVA/ritonavir and lopinavir/ritonavir are clinically equivalent.
Once-daily administration of LEXIVA plus ritonavir is not recommended for protease inhibitor-experienced patients. Through Week 48, 50% and 37% of subjects receiving LEXIVA 1,400 mg plus ritonavir 200 mg once daily had plasma HIV-1 RNA less than 400 copies per mL and less than 50 copies per mL, respectively.
Pediatric Trials
Three open-label trials in pediatric subjects aged at least 4 weeks to 18 years were conducted. In one trial (APV29005), twice-daily dosing regimens (LEXIVA with or without ritonavir) were evaluated in combination with other antiretroviral agents in pediatric subjects aged 2 to 18 years. In a second trial (APV20002), twice-daily dosing regimens (LEXIVA with ritonavir) were evaluated in combination with other antiretroviral agents in pediatric subjects aged at least 4 weeks to less than 2 years. A third trial (APV20003) evaluated once-daily dosing of LEXIVA with ritonavir; the pharmacokinetic data from this trial did not support a once-daily dosing regimen in any pediatric patient population.
- APV29005 LEXIVA
Twenty (18 therapy-naive and 2 therapy-experienced) pediatric subjects received LEXIVA Oral Suspension without ritonavir twice daily. At Week 24, 65% (13/20) achieved HIV-1 RNA less than 400 copies per mL, and the median increase from baseline in CD4+ cell count was 350 cells per mm3.
LEXIVA plus Ritonavir: Forty-nine protease inhibitor-naive and 40 protease inhibitor-experienced pediatric subjects received LEXIVA Oral Suspension or Tablets with ritonavir twice daily. At Week 24, 71% of protease inhibitor-naive (35/49) and 55% of protease inhibitor-experienced (22/40) subjects achieved HIV-1 RNA less than 400 copies per mL; median increases from baseline in CD4+ cell counts were 184 cells per mm3 and 150 cells per mm3 in protease inhibitor-naive and experienced subjects, respectively.
- APV20002
Fifty-four pediatric subjects (49 protease inhibitor-naive and 5 protease inhibitor-experienced) received LEXIVA Oral Suspension with ritonavir twice daily. At Week 24, 72% of subjects achieved HIV-1 RNA less than 400 copies per mL. The median increases from baseline in CD4+ cell counts were 400 cells per mm3 in subjects aged at least 4 weeks to less than 6 months and 278 cells per mm3 in subjects aged 6 months to 2 years.[1]
References
Adapted from the FDA Package Insert.