Human papillomavirus laboratory findings: Difference between revisions
(Category) |
m (Bot: Removing from Primary care) |
||
| Line 33: | Line 33: | ||
{{WH}} | {{WH}} | ||
{{WS}} | {{WS}} | ||
[[Category:Emergency mdicine]] | |||
[[Category:Disease]] | [[Category:Disease]] | ||
[[Category:Up-To-Date]] | [[Category:Up-To-Date]] | ||
[[Category:Infectious disease]] | [[Category:Infectious disease]] | ||
Latest revision as of 22:13, 29 July 2020
|
Human papillomavirus Microchapters |
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Human papillomavirus laboratory findings On the Web |
|
American Roentgen Ray Society Images of Human papillomavirus laboratory findings |
|
Risk calculators and risk factors for Human papillomavirus laboratory findings |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];Associate Editor(s)-in-Chief: Seyedmahdi Pahlavani, M.D. [2]
Overview
Various oncogenic types of HPV can be detected for cancer screening purposes. The development of HPV-induced cervical cancer is a slow process that generally takes many years. During this development phase, pre-cancerous cells can be detected by annual or semi-annual cervical cytology Papanicolaou screening, colloquially known as "Pap" smear testing.
Laboratory Findings
HPV infection can be detected and managed in various ways. HPV tests are available to detect oncogenic types of HPV infection and are used in the context of cervical cancer screening and management or follow-up of abnormal cervical cytology or histology.[1][2] Currently, 3 main methods to detect HPV are available:[3]
- HPV DNA testing
- HPV RNA testing
- Cellular markers screening
DNA based assays
They can be divided into:
- Target amplification methods (PCR with consensus or type-specific primers)
- Signal amplification methods (liquid-phase or in situ hybridization): Hybrid capture HPV DNA assay is the prototype in this method and can detect 13 different types of high risk HPVs.[3]
RNA based assays
These tests look for expression of E6 and/or E7 RNA in HPV genome and provide higher sensitivity and specificity than DNA based testings. Persistent expression of E6 and E7 could serve as an indicator of progression from intraepithelial neoplasia to invasive cancer.[4][5][3]
Cellular markers screening
They are not FDA approved currently, but studies demonstrated high sensitivity to detect cervical dysplasia.[6]
Pap Smear
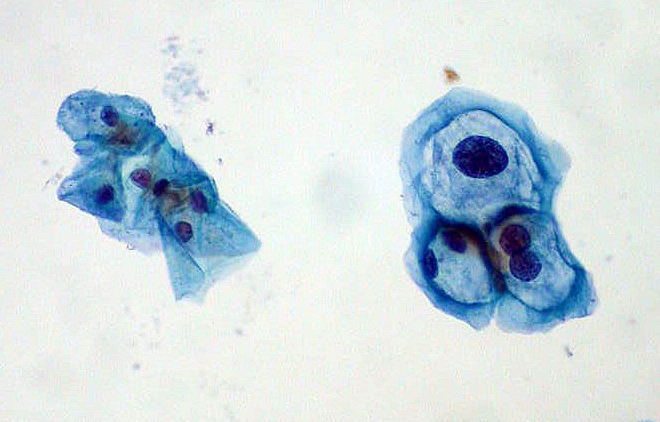
The Pap test is an effective strategy for reducing the risk of invasive cervical cancer. The Pap test involves taking cells from the cervix and putting them on a small glass slide and examining them under a microscope to look for abnormal cells. This method is 70% to 80% effective in detecting HPV-caused cellular abnormalities. A more sensitive method is a “Thin Prep,” in which the cells from the cervix are placed in a liquid solution. This test is 85% to 95% effective in detecting HPV-caused cellular abnormalities. The last Pap test method is mainly used on women over 30. It is a combination Pap-HPV DNA test. If this test comes back negative women can usually wait 3 years before having the test done again. Detailed inspection of the cervix by colposcopy may be indicated if abnormal cells are detected by routine Pap smear. A frequently occurring example of an abnormal cell found in association with HPV is the koilocyte.[7](See figure.)
-
ThinPrep Pap smear with group of normal cervical cells on left and HPV-infected cells on right. The HPV-infected cells show features typical of koilocytes: enlarged (x2 or x3) nuclei and hyperchromasia.
References
- ↑ Schiffman M, Wentzensen N, Wacholder S, Kinney W, Gage JC, Castle PE (2011). "Human papillomavirus testing in the prevention of cervical cancer". J. Natl. Cancer Inst. 103 (5): 368–83. doi:10.1093/jnci/djq562. PMC 3046952. PMID 21282563.
- ↑ "WHO | WHO HPV LabNet".
- ↑ 3.0 3.1 3.2 Chan PK, Picconi MA, Cheung TH, Giovannelli L, Park JS (2012). "Laboratory and clinical aspects of human papillomavirus testing". Crit Rev Clin Lab Sci. 49 (4): 117–36. doi:10.3109/10408363.2012.707174. PMC 3469219. PMID 22913405.
- ↑ Sotlar K, Selinka HC, Menton M, Kandolf R, Bültmann B (1998). "Detection of human papillomavirus type 16 E6/E7 oncogene transcripts in dysplastic and nondysplastic cervical scrapes by nested RT-PCR". Gynecol. Oncol. 69 (2): 114–21. doi:10.1006/gyno.1998.4994. PMID 9600817.
- ↑ Lie AK, Risberg B, Borge B, Sandstad B, Delabie J, Rimala R, Onsrud M, Thoresen S (2005). "DNA- versus RNA-based methods for human papillomavirus detection in cervical neoplasia". Gynecol. Oncol. 97 (3): 908–15. doi:10.1016/j.ygyno.2005.02.026. PMID 15943992.
- ↑ Ikenberg H, Bergeron C, Schmidt D, Griesser H, Alameda F, Angeloni C, Bogers J, Dachez R, Denton K, Hariri J, Keller T, von Knebel Doeberitz M, Neumann HH, Puig-Tintore LM, Sideri M, Rehm S, Ridder R (2013). "Screening for cervical cancer precursors with p16/Ki-67 dual-stained cytology: results of the PALMS study". J. Natl. Cancer Inst. 105 (20): 1550–7. doi:10.1093/jnci/djt235. PMC 3814411. PMID 24096620.
- ↑ Sopracordevole F, Mancioli F, Clemente N, De Piero G, Buttignol M, Giorda G, Ciavattini A (2015). "Abnormal Pap Smear and Diagnosis of High-Grade Vaginal Intraepithelial Neoplasia: A Retrospective Cohort Study". Medicine (Baltimore). 94 (42): e1827. doi:10.1097/MD.0000000000001827. PMC 4620759. PMID 26496321.