Wild-type (senile) amyloidosis pathophysiology: Difference between revisions
No edit summary |
|||
| (14 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{Wild-type (senile) amyloidosis}} | {{Wild-type (senile) amyloidosis}} | ||
{{CMG}}{{AE}}{{ | {{CMG}}{{AE}}{{Sab}} | ||
==Overview== | ==Overview== | ||
[[Amyloid]] is an abnormal insoluble [[extracellular]] [[protein]] that deposits in the different [[Tissue (biology)|tissues]] and causes organ dysfunction and a wide variety of clinical [[Syndrome|syndromes]]. Wild-type (senile) amyloidosis is a type of [[Amyloidosis|systemic amyloidosis]] as [[Transthyretin|transthyretin (TTR)]] deposits can be found throughout the [[Human body|body]]. [[Transthyretin|TTR]] results in [[Pathology|pathologies]] due to misfolding, breaking apart, and deposition of the [[amyloid]] fibrils in healthy [[Tissue (biology)|tissue]]. The condition mainly affects the [[heart]]. However, other [[organ systems]], such as the [[Nervous system|nervous]] and [[Musculoskeletal system|musculoskeletal systems]], can also be involved. There are no [[genes]] implicated in the causality of wild-type (senile) amyloidosis. [[Ageing|Aging]] is very strongly associated with wild-type (senile) amyloidosis. | |||
==Pathophysiology== | ==Pathophysiology== | ||
*[[Amyloid]] is an abnormal insoluble [[extracellular]] [[protein]] that deposits in the different tissues and causes organ dysfunction and a wide variety of clinical [[Syndrome|syndromes]].<ref name="pmid26719234">{{cite journal |vauthors=Wechalekar AD, Gillmore JD, Hawkins PN |title=Systemic amyloidosis |journal=Lancet |volume=387 |issue=10038 |pages=2641–2654 |date=June 2016 |pmid=26719234 |doi=10.1016/S0140-6736(15)01274-X |url=}}</ref> | |||
*These abnormal [[Amyloid|amyloids]] are derived from misfolding and aggregation of normally soluble [[Protein|proteins]]. | |||
*[[Amyloid]] deposition can disrupt [[Tissue (biology)|tissue]] structure of involved [[Organ (anatomy)|organ]] and consequently leads to [[organ failure]].<ref name="pmid267192342">{{cite journal |vauthors=Wechalekar AD, Gillmore JD, Hawkins PN |title=Systemic amyloidosis |journal=Lancet |volume=387 |issue=10038 |pages=2641–2654 |date=June 2016 |pmid=26719234 |doi=10.1016/S0140-6736(15)01274-X |url=}}</ref> | |||
===Systemic Amyloidosis=== | |||
*In [[Amyloidosis|systemic amyloidosis]], [[amyloid]] gradually accumulates and [[amyloid]] deposition is widespread in the [[viscera]], [[blood vessel]] walls, and different [[Connective tissue|connective tissues]].<ref name="pmid23227278">{{cite journal |vauthors=Baker KR, Rice L |title=The amyloidoses: clinical features, diagnosis and treatment |journal=Methodist Debakey Cardiovasc J |volume=8 |issue=3 |pages=3–7 |date=2012 |pmid=23227278 |pmc=3487569 |doi= |url=}}</ref><ref name="pmid16409147">{{cite journal |vauthors=Pepys MB |title=Amyloidosis |journal=Annu. Rev. Med. |volume=57 |issue= |pages=223–41 |date=2006 |pmid=16409147 |doi=10.1146/annurev.med.57.121304.131243 |url=}}</ref> | |||
===Pathogenesis=== | ===Pathogenesis=== | ||
* Wild-type (senile) amyloidosis is a type of [[Amyloidosis|systemic amyloidosis]] as [[Transthyretin|transthyretin (TTR)]] deposits can be found throughout the [[Human body|body]].<ref>{{Cite journal | |||
| author = [[Ilia G. Halatchev]], [[Jingsheng Zheng]] & [[Jiafu Ou]] | |||
| title = Wild-type transthyretin cardiac amyloidosis (ATTRwt-CA), previously known as senile cardiac amyloidosis: clinical presentation, diagnosis, management and emerging therapies | |||
| journal = [[Journal of thoracic disease]] | |||
| volume = 10 | |||
| issue = 3 | |||
| pages = 2034–2045 | |||
| year = 2018 | |||
| month = March | |||
| doi = 10.21037/jtd.2018.03.134 | |||
| pmid = 29707360 | |||
}}</ref> | |||
* The culprit [[protein]] responsible for the disease is [[Transthyretin|TTR]] and it is deposited in the non-[[Mutation|mutated]] form, hence the name "wild-type". | |||
*[[Transthyretin|TTR]] results in [[Pathology|pathologies]] due to misfolding, breaking apart, and deposition of the [[amyloid]] fibrils in healthy [[Tissue (biology)|tissue]]. | |||
* The normal [[Transthyretin|TTR protein]], compared with the [[Mutation|mutated]] form, is less likely to get deposited and cause [[pathology]]. | |||
*This is believed to be the reason as to why this condition almost always affects the [[Old age|elderly]] (65 years of age or older). | |||
*The condition mainly affects the [[heart]]. However, other [[organ systems]], such as the [[Nervous system|nervous]] and [[Musculoskeletal system|musculoskeletal systems]], can also be involved. | |||
== Genetics == | |||
* There are no [[genes]] implicated in the causality of wild-type (senile) amyloidosis. | |||
== Associated Conditions == | |||
* | *[[Ageing|Aging]] is very strongly associated with wild-type (senile) amyloidosis. | ||
==Gross Pathology== | |||
Cardiac amyloid deposits are most commonly seen in the [[myocardium]], but can also be seen in the [[atrium (heart)|atria]], [[pericardium]], [[endocardium]] and microvasculature. | |||
* On gross examination, the myocardium is thicker, firm and rubbery in consistency. More than half of myocardium involvement is common in the AL type of cardiac amyloidosis. | |||
* The size of the ventricular cavity remains unchanged, however filling of the ventricles is restricted (causing [[restrictive cardiomyopathy]]) because of stiffening of the ventricular wall as a result of deposition of amyloid material. | |||
* [[Pericardial effusion]] and valvular dysfunction is common from pericardial and endocardial involvement. [[Intracardiac thrombus]] formation is frequently seen and may result in fatal [[thromboembolism]].<ref name="pmid22583098">{{cite journal |author=Nakagawa M, Tojo K, Sekijima Y, Yamazaki KH, Ikeda S |title=Arterial thromboembolism in senile systemic amyloidosis: report of two cases |journal=[[Amyloid : the International Journal of Experimental and Clinical Investigation : the Official Journal of the International Society of Amyloidosis]] |volume=19 |issue=2 |pages=118–21 |year=2012 |month=June |pmid=22583098 |doi=10.3109/13506129.2012.685131 |url=}}</ref><ref name="pmid21893485">{{cite journal |author=Van de Veire NR, Dierick J, De Sutter J |title=Intracardiac emboli as first presentation of cardiac AL amyloidosis |journal=[[European Heart Journal]] |volume=33 |issue=7 |pages=818 |year=2012 |month=April |pmid=21893485 |pmc=3345559 |doi=10.1093/eurheartj/ehr330 |url=}}</ref><ref name="pmid17984380">{{cite journal |author=Feng D, Edwards WD, Oh JK, ''et al.'' |title=Intracardiac thrombosis and embolism in patients with cardiac amyloidosis |journal=[[Circulation]] |volume=116 |issue=21 |pages=2420–6 |year=2007 |month=November |pmid=17984380 |doi=10.1161/CIRCULATIONAHA.107.697763 |url=}}</ref> | |||
===Images=== | |||
[http://www.peir.net Images shown below are courtesy of Professor Peter Anderson and published with permission. © PEIR, University of Alabama at Birmingham, Department of Pathology] | |||
{| align="left" | |||
|-valign="top" | |||
| [[Image:LA Amyloid.jpg|thumb|325 px|Amyloidosis Lesion In Left Atrium: Gross natural color view of a diagnostic lesion]] | |||
| [[Image:LA Amyloid 2.jpg|thumb|325 px|Amyloidosis Lesion In Left Atrium: Gross natural color close-up]] | |||
|} | |||
{| align="left" | |||
|-valign="top" | |||
| [[Image:Amyloidosis LA nodules.jpg|thumb|325 px|Amyloidosis, left atrium, endocardial nodules]] | |||
| [[Image:Amyloidosis endocardial nodules.jpg|thumb|325 px|Amyloidosis, left atrium, endocardial nodules]] | |||
| [[Image:Amyloidosis and LVH.jpg|thumb|325 px|Amyloidosis and left ventricular hypertrophy]] | |||
|} | |||
<br style="clear:left"> | |||
==Microscopic Pathology== | |||
* Under light microscope, extracellular deposits of hyaline like amyloid material are evident. Resultant myocardial [[fibrosis]] restricts the movement of the ventricle, compromising complete filling of the ventricle during diastole. | |||
* Amyloid deposits are also seen in the vasculature, particularly the microvasculature thereby sparing the large epicardial vessels. This leads to [[myocardial ischemia]] and tissue [[infarction]]. | |||
{| align="left" | |||
|-valign="top" | |||
| [[Image:Amyloidosis Kongored.jpg|thumb|325 px|Heart: Perivascular amyloid, amyloidosis, congo red showing birefringence]] | |||
| [[Image:Perivas Amyloid.jpg|thumb|325 px|Heart: Perivascular amyloid, amyloidosis (Hematoxylin and eosin staining)]] | |||
| [[Image:Aldehyd Fuchsin.jpg|thumb|325 px|Heart: Amyloidosis, aldehyde fuchsin stain]] | |||
|} | |||
<br style="clear:left"> | |||
==References== | ==References== | ||
Latest revision as of 18:28, 20 December 2019
|
Wild-type (senile) amyloidosis Microchapters |
|
Differentiating Wild-type (senile) amyloidosis from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Wild-type (senile) amyloidosis pathophysiology On the Web |
|
American Roentgen Ray Society Images of Wild-type (senile) amyloidosis pathophysiology |
|
Risk calculators and risk factors for Wild-type (senile) amyloidosis pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]Associate Editor(s)-in-Chief: Sabawoon Mirwais, M.B.B.S, M.D.[2]
Overview
Amyloid is an abnormal insoluble extracellular protein that deposits in the different tissues and causes organ dysfunction and a wide variety of clinical syndromes. Wild-type (senile) amyloidosis is a type of systemic amyloidosis as transthyretin (TTR) deposits can be found throughout the body. TTR results in pathologies due to misfolding, breaking apart, and deposition of the amyloid fibrils in healthy tissue. The condition mainly affects the heart. However, other organ systems, such as the nervous and musculoskeletal systems, can also be involved. There are no genes implicated in the causality of wild-type (senile) amyloidosis. Aging is very strongly associated with wild-type (senile) amyloidosis.
Pathophysiology
- Amyloid is an abnormal insoluble extracellular protein that deposits in the different tissues and causes organ dysfunction and a wide variety of clinical syndromes.[1]
- These abnormal amyloids are derived from misfolding and aggregation of normally soluble proteins.
- Amyloid deposition can disrupt tissue structure of involved organ and consequently leads to organ failure.[2]
Systemic Amyloidosis
- In systemic amyloidosis, amyloid gradually accumulates and amyloid deposition is widespread in the viscera, blood vessel walls, and different connective tissues.[3][4]
Pathogenesis
- Wild-type (senile) amyloidosis is a type of systemic amyloidosis as transthyretin (TTR) deposits can be found throughout the body.[5]
- The culprit protein responsible for the disease is TTR and it is deposited in the non-mutated form, hence the name "wild-type".
- TTR results in pathologies due to misfolding, breaking apart, and deposition of the amyloid fibrils in healthy tissue.
- The normal TTR protein, compared with the mutated form, is less likely to get deposited and cause pathology.
- This is believed to be the reason as to why this condition almost always affects the elderly (65 years of age or older).
- The condition mainly affects the heart. However, other organ systems, such as the nervous and musculoskeletal systems, can also be involved.
Genetics
- There are no genes implicated in the causality of wild-type (senile) amyloidosis.
Associated Conditions
- Aging is very strongly associated with wild-type (senile) amyloidosis.
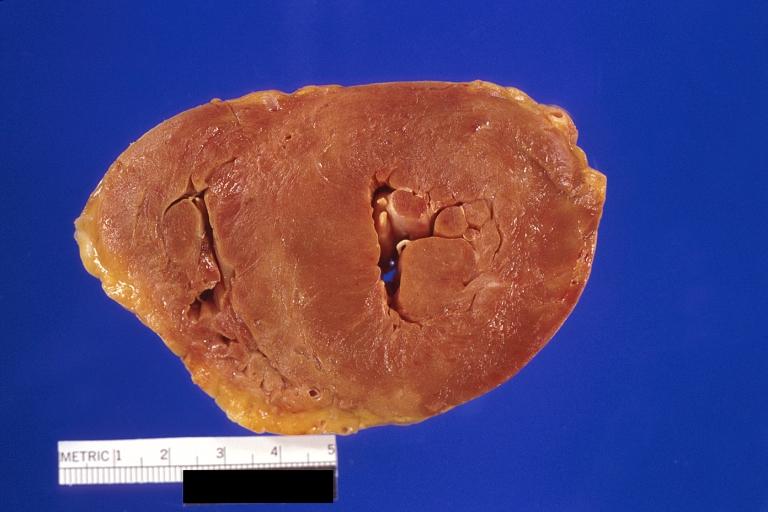
Gross Pathology
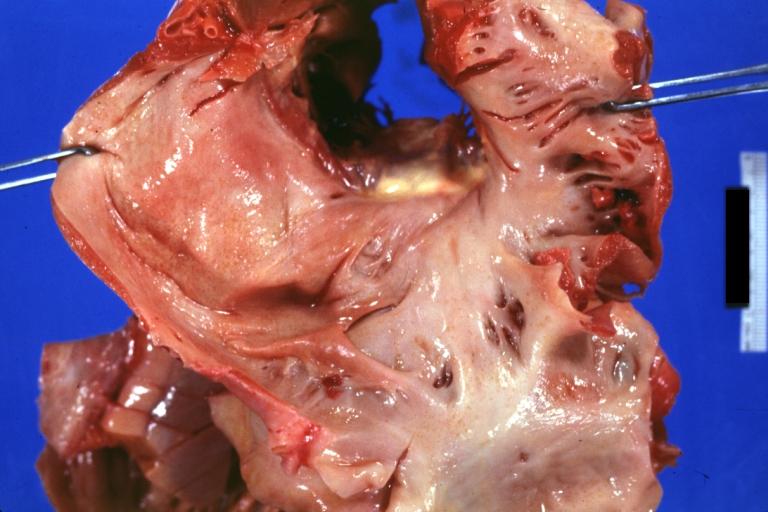
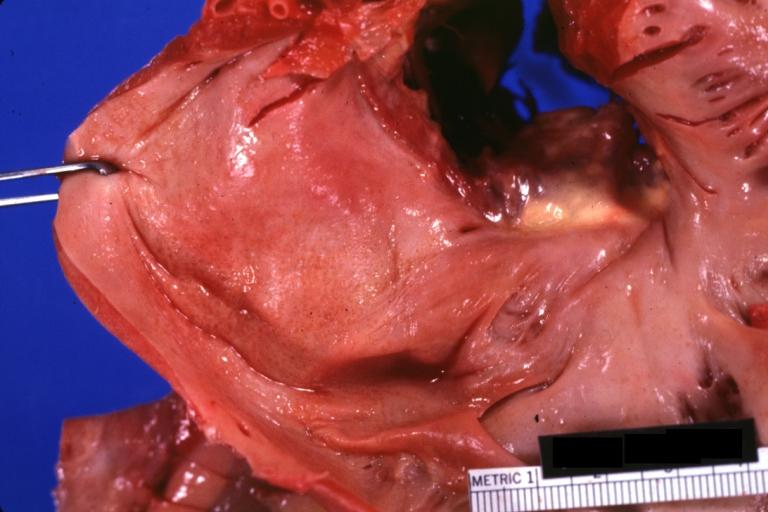
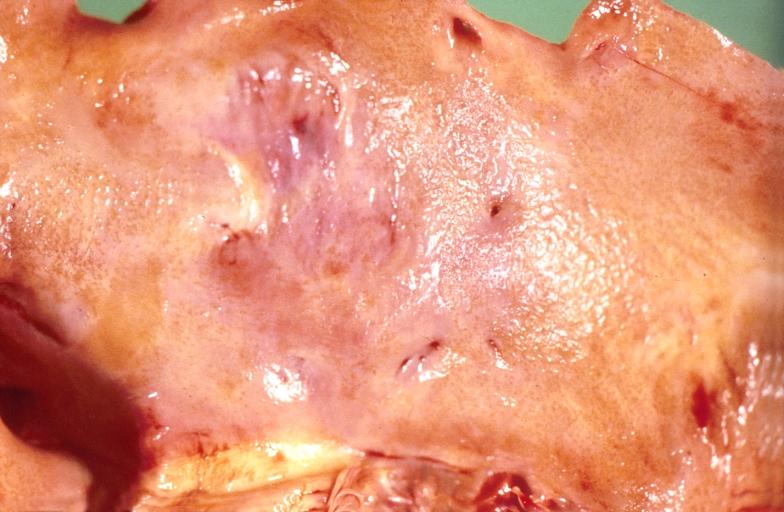
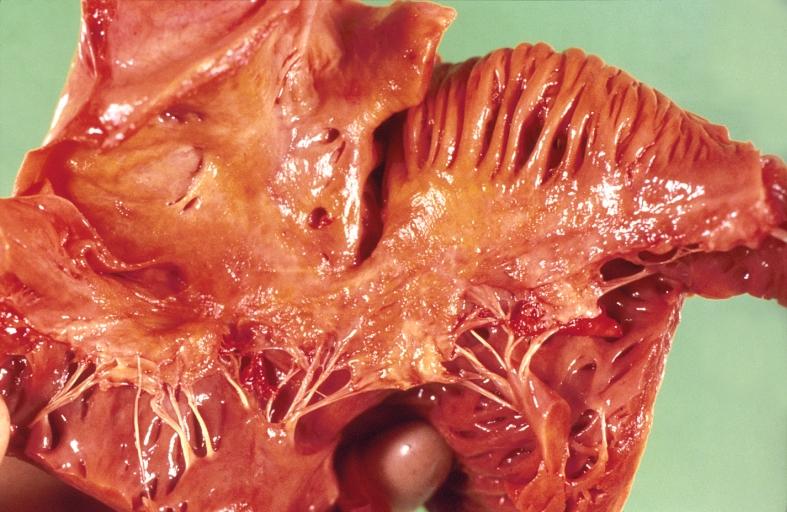
Cardiac amyloid deposits are most commonly seen in the myocardium, but can also be seen in the atria, pericardium, endocardium and microvasculature.
- On gross examination, the myocardium is thicker, firm and rubbery in consistency. More than half of myocardium involvement is common in the AL type of cardiac amyloidosis.
- The size of the ventricular cavity remains unchanged, however filling of the ventricles is restricted (causing restrictive cardiomyopathy) because of stiffening of the ventricular wall as a result of deposition of amyloid material.
- Pericardial effusion and valvular dysfunction is common from pericardial and endocardial involvement. Intracardiac thrombus formation is frequently seen and may result in fatal thromboembolism.[6][7][8]
Images
 |
 |
 |
 |
 |
Microscopic Pathology
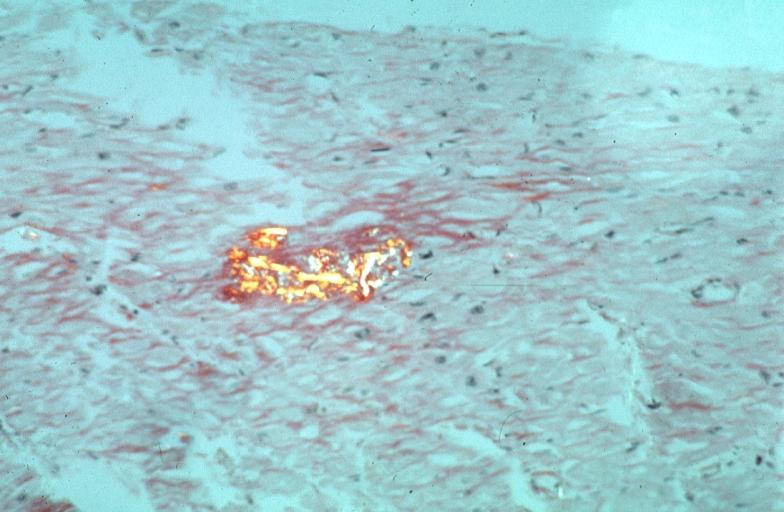
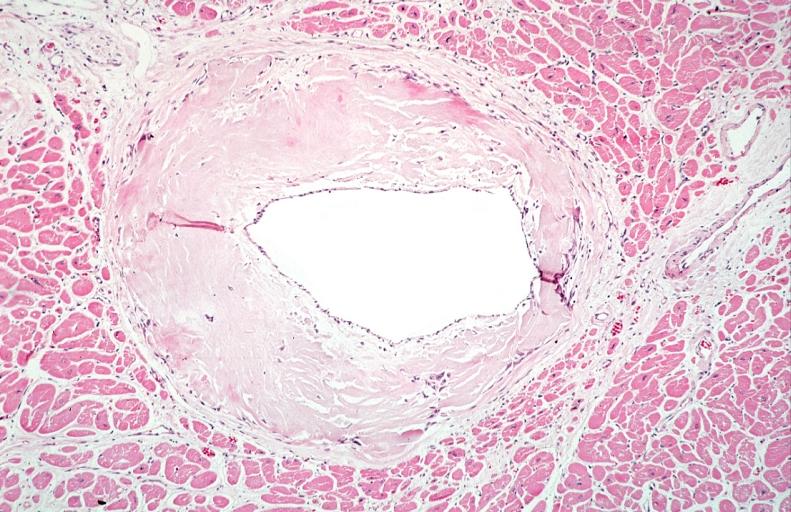
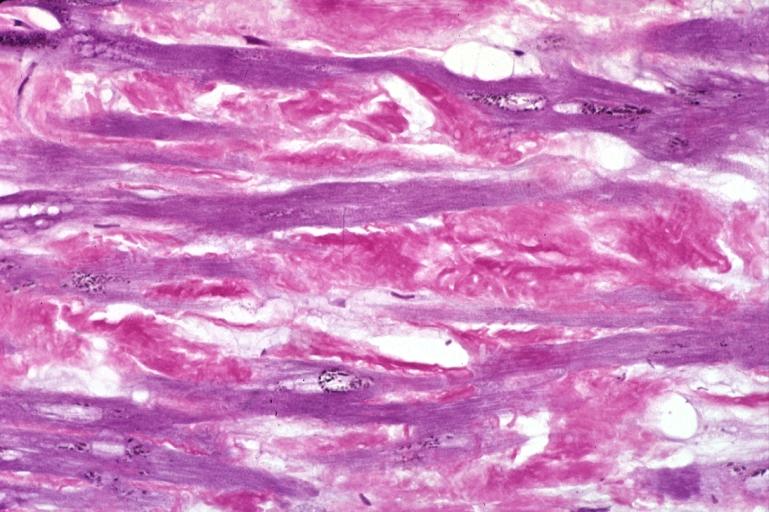
- Under light microscope, extracellular deposits of hyaline like amyloid material are evident. Resultant myocardial fibrosis restricts the movement of the ventricle, compromising complete filling of the ventricle during diastole.
- Amyloid deposits are also seen in the vasculature, particularly the microvasculature thereby sparing the large epicardial vessels. This leads to myocardial ischemia and tissue infarction.
 |
 |
 |
References
- ↑ Wechalekar AD, Gillmore JD, Hawkins PN (June 2016). "Systemic amyloidosis". Lancet. 387 (10038): 2641–2654. doi:10.1016/S0140-6736(15)01274-X. PMID 26719234.
- ↑ Wechalekar AD, Gillmore JD, Hawkins PN (June 2016). "Systemic amyloidosis". Lancet. 387 (10038): 2641–2654. doi:10.1016/S0140-6736(15)01274-X. PMID 26719234.
- ↑ Baker KR, Rice L (2012). "The amyloidoses: clinical features, diagnosis and treatment". Methodist Debakey Cardiovasc J. 8 (3): 3–7. PMC 3487569. PMID 23227278.
- ↑ Pepys MB (2006). "Amyloidosis". Annu. Rev. Med. 57: 223–41. doi:10.1146/annurev.med.57.121304.131243. PMID 16409147.
- ↑ Ilia G. Halatchev, Jingsheng Zheng & Jiafu Ou (2018). "Wild-type transthyretin cardiac amyloidosis (ATTRwt-CA), previously known as senile cardiac amyloidosis: clinical presentation, diagnosis, management and emerging therapies". Journal of thoracic disease. 10 (3): 2034–2045. doi:10.21037/jtd.2018.03.134. PMID 29707360. Unknown parameter
|month=ignored (help) - ↑ Nakagawa M, Tojo K, Sekijima Y, Yamazaki KH, Ikeda S (2012). "Arterial thromboembolism in senile systemic amyloidosis: report of two cases". Amyloid : the International Journal of Experimental and Clinical Investigation : the Official Journal of the International Society of Amyloidosis. 19 (2): 118–21. doi:10.3109/13506129.2012.685131. PMID 22583098. Unknown parameter
|month=ignored (help) - ↑ Van de Veire NR, Dierick J, De Sutter J (2012). "Intracardiac emboli as first presentation of cardiac AL amyloidosis". European Heart Journal. 33 (7): 818. doi:10.1093/eurheartj/ehr330. PMC 3345559. PMID 21893485. Unknown parameter
|month=ignored (help) - ↑ Feng D, Edwards WD, Oh JK; et al. (2007). "Intracardiac thrombosis and embolism in patients with cardiac amyloidosis". Circulation. 116 (21): 2420–6. doi:10.1161/CIRCULATIONAHA.107.697763. PMID 17984380. Unknown parameter
|month=ignored (help)