Cholangiocarcinoma pathophysiology: Difference between revisions
No edit summary |
|||
| (37 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{Cholangiocarcinoma}} | {{Cholangiocarcinoma}} | ||
{{CMG}};{{AE}}{{ | |||
{{CMG}}; {{AE}} {{F.K}}, {{Anmol}} | |||
==Overview== | ==Overview== | ||
The [[epithelial cell]] lining the [[bile ducts]] are called [[cholangiocytes]]. The malignant transformsation of cholangiocytes leads to cholangiocarcinoma. [[Malignant]] [[transformation]] of cholangiocytes into cholangiocarcinoma include [[hyperplasia]], [[metaplasia]] and dysplasia. Biliary intraepithelial [[neoplasia]] is believed to be the initial lesion of cholangiocarcinoma, particularly in patients with [[hepatolithiasis]] in [[bile ducts]]. Gross pathologic features characteristic to intrahepatic cholangiocarcinoma are divided in three subtypes and include mass forming type, periductal infiltrating type, and intraductal growth type. On microscopic pathology, characteristic findings of cholangiocarcinoma include [[Cuboidal epithelia|cuboidal]] or columnar [[mucin]] producing cells and dense fibrous (desmoplastic) [[stroma]]. | |||
==Pathophysiology== | ==Pathophysiology== | ||
* | ===Pathogenesis=== | ||
* | *The [[epithelial cell]] lining the bile ducts are called [[cholangiocytes]]. The malignant transformsation of [[cholangiocytes]] leads to cholangiocarcinoma.<ref name="FavaLorenzini2012">{{cite journal|last1=Fava|first1=G.|last2=Lorenzini|first2=I.|title=Molecular Pathogenesis of Cholangiocarcinoma|journal=International Journal of Hepatology|volume=2012|year=2012|pages=1–7|issn=2090-3448|doi=10.1155/2012/630543}}</ref> | ||
* | *Malignant transformation of [[cholangiocytes]] into cholangiocarcinoma include following stages:<ref name="targeting">{{cite journal |author=Sirica A |title=Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy |journal=Hepatology |volume=41 |issue=1 |pages=5–15 |year=2005 |id=PMID 15690474}}</ref> | ||
* | *#[[Hyperplasia]] | ||
* | *#[[Metaplasia]] | ||
* | *#[[Dysplasia]] | ||
* | *#Frank carcinoma | ||
*Progression of [[malignancy]] is believed to be due to:<ref name="targeting" /><ref>{{cite journal |author=Holzinger F, Z'graggen K, Büchler M |title=Mechanisms of biliary carcinogenesis: a pathogenetic multi-stage cascade towards cholangiocarcinoma |journal=Ann Oncol |volume=10 Suppl 4 |issue= |pages=122-6 |year= |id=PMID 10436802}}</ref><ref>{{cite journal |author=Gores G |title=Cholangiocarcinoma: current concepts and insights |journal=Hepatology |volume=37 |issue=5 |pages=961-9 |year=2003 |id=PMID 12717374}}</ref> | |||
**[[Inflammation]] | |||
**[[Obstruction]] of [[bile ducts]] | |||
**[[Biliary]] intraepithelia [[neoplasia]] | |||
*[[Biliary]] intraepithelial neoplasia is believed to be the initial lesion of cholangiocarcinoma, particularly in patients with [[hepatolithiasis]] in bile ducts. | |||
==Genetics== | |||
<div style="text-align: center">'''Algorithm showing molecular mechanism of cholangiocarcinoma'''<ref name="pmid21691113">{{cite journal |vauthors=Wadsworth CA, Dixon PH, Wong JH, Chapman MH, McKay SC, Sharif A, Spalding DR, Pereira SP, Thomas HC, Taylor-Robinson SD, Whittaker J, Williamson C, Khan SA |title=Genetic factors in the pathogenesis of cholangiocarcinoma |journal=Dig Dis |volume=29 |issue=1 |pages=93–7 |year=2011 |pmid=21691113 |pmc=3696362 |doi=10.1159/000324688 |url=}}</ref><ref name="pmid25332972">{{cite journal |vauthors=Maroni L, Pierantonelli I, Banales JM, Benedetti A, Marzioni M |title=The significance of genetics for cholangiocarcinoma development |journal=Ann Transl Med |volume=1 |issue=3 |pages=28 |year=2013 |pmid=25332972 |pmc=4200671 |doi=10.3978/j.issn.2305-5839.2012.10.04 |url=}}</ref><ref name="pmid25966424">{{cite journal |vauthors=Kongpetch S, Jusakul A, Ong CK, Lim WK, Rozen SG, Tan P, Teh BT |title=Pathogenesis of cholangiocarcinoma: From genetics to signalling pathways |journal=Best Pract Res Clin Gastroenterol |volume=29 |issue=2 |pages=233–44 |year=2015 |pmid=25966424 |doi=10.1016/j.bpg.2015.02.002 |url=}}</ref></div> | |||
<br> | |||
{{Family tree/start}} | |||
{{Family tree| | | | | | | | A01 | | | | | | A01=Cholangiocytes}} | |||
{{Family tree| | | | | | | | |!| | | | | | | }} | |||
{{Family tree| | | | | | | | B01 | | | | | | B01=Proinflammatory cytokines ([[TNF-α]] and [[IL-6]])}} | |||
{{Family tree| | | | | | | | |!| | | | | | | }} | |||
{{Family tree| | | | C01 | | C02 | | C03 | | C01=Several cytokines )|C02=Stimulates the expression of inducible nitric oxide synthase (iNOS) and enhancing NO production|C03=Reactive oxygen species}} | |||
{{Family tree| | | | | |`|v|'| |`|v|'| | | | }} | |||
{{Family tree| | D01 | | |!| | | D02 | | | | D01=[[EGFR]] (epidermal growth factor receptor) family, specifically the tyrosine kinase ErbB-2 ([[HER2/neu]]|D02=Inhibit DNA repair mechanism}} | |||
{{Family tree| |!|!| | | |!| | | |!| | | | | }} | |||
{{Family tree| |!|!|,|-|-|(| | | |!| | | | | }} | |||
{{Family tree| |!| E02 | | E01 | |!| | | | | E01=Nitrosylation of caspase|E02=Stimulation of [[Cyclooxygenase|cyclooxygenase 2]] (COX-2)}} | |||
{{Family tree| |!| |!| | | |!| | |!| | | | | }} | |||
{{Family tree| E03 |!| | | |!| | |!| | | | | E03=Increased invasiveness, proliferation, and mobility of cholangiocytes}} | |||
{{Family tree| | | |!| | | |!| | |!| | | | | }} | |||
{{Family tree| | | F01 | | |!| | |!| | | | | F01=Prostaglandin E2 production(Rate limitimg step)}} | |||
{{Family tree| | | |`|-|v|-|'| | |!| | | | | }} | |||
{{Family tree| | | | | |!| | | | |!| | | | | }} | |||
{{Family tree| | | |,|-|^|-|.| | |!| | | | | }} | |||
{{Family tree| | | G01 | | G02 | | G03 | | | | G01=Activate cell cycle of cholangiocytes|G02=Inhibit apoptosis of cholangiocytes|G03=Promotes mutagenesis}} | |||
{{Family tree/end}} | |||
==Gross Pathology== | ==Gross Pathology== | ||
Gross pathologic features characteristic to intrahepatic cholangiocarcinoma are divided in three subtypes and include:<ref name="pmid21191517">{{cite journal |vauthors=Nakanuma Y, Sato Y, Harada K, Sasaki M, Xu J, Ikeda H |title=Pathological classification of intrahepatic cholangiocarcinoma based on a new concept |journal=World J Hepatol |volume=2 |issue=12 |pages=419–27 |year=2010 |pmid=21191517 |pmc=3010511 |doi=10.4254/wjh.v2.i12.419 |url=}}</ref><ref name="pmid21808282">{{cite journal |vauthors=Blechacz B, Komuta M, Roskams T, Gores GJ |title=Clinical diagnosis and staging of cholangiocarcinoma |journal=Nat Rev Gastroenterol Hepatol |volume=8 |issue=9 |pages=512–22 |year=2011 |pmid=21808282 |pmc=3331791 |doi=10.1038/nrgastro.2011.131 |url=}}</ref><ref name="pmid28261592">{{cite journal |vauthors=Vijgen S, Terris B, Rubbia-Brandt L |title=Pathology of intrahepatic cholangiocarcinoma |journal=Hepatobiliary Surg Nutr |volume=6 |issue=1 |pages=22–34 |year=2017 |pmid=28261592 |pmc=5332210 |doi=10.21037/hbsn.2016.11.04 |url=}}</ref> | |||
*'''Mass-forming type''' | |||
**Nodular lesion or mass in the hepatic [[parenchyma]] | |||
**Gray to gray-white, firm and solid carcinoma | |||
*'''Periductal infiltrating type''' | |||
**Spreading of the [[carcinoma]] along the portal tracts with [[stricture]] of the affected [[bile ducts]] | |||
**Dilatation of the peripheral [[bile ducts]] | |||
*'''Intraductal growth type''' | |||
**Polypoid or papillary tumor within the variably dilated bile duct lumen | |||
**[[Malignant]] progression of an intraductal papillary [[neoplasm]] of the [[bile duct]] | |||
{| | |||
Intrahepatic | | | ||
[[File:Cholangiocarcinoma.png|thumb|400px|left|Intrahepatic cholangiocarcinoma [https://commons.wikimedia.org/wiki/File:Cholangiocarcinoma.png Source: By Banchob Sripa, via Wikimedia Commons]<ref name="urlFile:Cholangiocarcinoma.png - Wikimedia Commons">{{cite web |url=https://commons.wikimedia.org/wiki/File:Cholangiocarcinoma.png |title=File:Cholangiocarcinoma.png - Wikimedia Commons |format= |work= |accessdate=}}</ref>]] | |||
| | |||
[[File:CCA Cholangiocarcinoma.jpg|thumb|400px|left|Cholangiocarcinoma [https://commons.wikimedia.org/wiki/File:CCA_Cholangiocarcinoma.jpg Source: By Sripa B, Kaewkes S, Sithithaworn P, Mairiang E, Laha T, et al, via Wikimedia Commons]<ref name="urlFile:CCA Cholangiocarcinoma.jpg - Wikimedia Commons">{{cite web |url=https://commons.wikimedia.org/wiki/File:CCA_Cholangiocarcinoma.jpg |title=File:CCA Cholangiocarcinoma.jpg - Wikimedia Commons |format= |work= |accessdate=}}</ref>]] | |||
|} | |||
<br style="clear:left" /> | |||
==Microscopic Pathology== | ==Microscopic Pathology== | ||
On microscopic pathology, characteristic findings of cholangiocarcinoma include: | |||
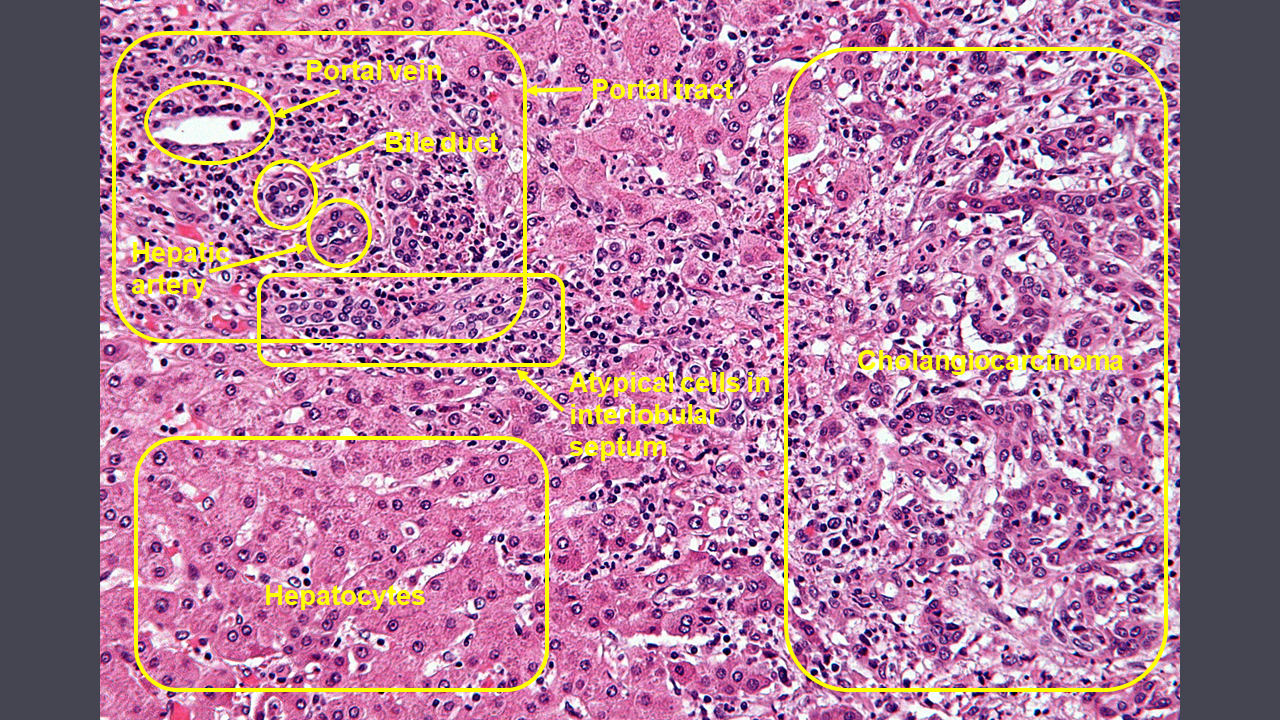
* | *[[Cuboidal]] or columnar [[mucin]] producing cells | ||
* | *Dense fibrous (desmoplastic) [[stroma]] | ||
[[ | [[File:Cholangiocarcinoma - histopathology.png|thumb|800px|left|Cholangiocarcinoma histopathology [https://commons.wikimedia.org/wiki/File:Cholangiocarcinoma_-_high_mag.jpg Source: By Nephron (Own work), via Wikimedia Commons]<ref name="urlFile:Cholangiocarcinoma - high mag.jpg - Wikimedia Commons">{{cite web |url=https://commons.wikimedia.org/wiki/File:Cholangiocarcinoma_-_high_mag.jpg |title=File:Cholangiocarcinoma - high mag.jpg - Wikimedia Commons |format= |work= |accessdate=}}</ref>]] | ||
<br style="clear:left" /> | |||
==References== | ==References== | ||
{{ | {{Reflist|2}} | ||
{{WH}} | {{WH}} | ||
{{WS}} | {{WS}} | ||
[[Category: (name of the system)]] | |||
Latest revision as of 20:18, 6 February 2018
|
Cholangiocarcinoma Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Cholangiocarcinoma pathophysiology On the Web |
|
American Roentgen Ray Society Images of Cholangiocarcinoma pathophysiology |
|
Risk calculators and risk factors for Cholangiocarcinoma pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Farima Kahe M.D. [2], Anmol Pitliya, M.B.B.S. M.D.[3]
Overview
The epithelial cell lining the bile ducts are called cholangiocytes. The malignant transformsation of cholangiocytes leads to cholangiocarcinoma. Malignant transformation of cholangiocytes into cholangiocarcinoma include hyperplasia, metaplasia and dysplasia. Biliary intraepithelial neoplasia is believed to be the initial lesion of cholangiocarcinoma, particularly in patients with hepatolithiasis in bile ducts. Gross pathologic features characteristic to intrahepatic cholangiocarcinoma are divided in three subtypes and include mass forming type, periductal infiltrating type, and intraductal growth type. On microscopic pathology, characteristic findings of cholangiocarcinoma include cuboidal or columnar mucin producing cells and dense fibrous (desmoplastic) stroma.
Pathophysiology
Pathogenesis
- The epithelial cell lining the bile ducts are called cholangiocytes. The malignant transformsation of cholangiocytes leads to cholangiocarcinoma.[1]
- Malignant transformation of cholangiocytes into cholangiocarcinoma include following stages:[2]
- Hyperplasia
- Metaplasia
- Dysplasia
- Frank carcinoma
- Progression of malignancy is believed to be due to:[2][3][4]
- Inflammation
- Obstruction of bile ducts
- Biliary intraepithelia neoplasia
- Biliary intraepithelial neoplasia is believed to be the initial lesion of cholangiocarcinoma, particularly in patients with hepatolithiasis in bile ducts.
Genetics
| Cholangiocytes | |||||||||||||||||||||||||||||||
| Proinflammatory cytokines (TNF-α and IL-6) | |||||||||||||||||||||||||||||||
| Several cytokines ) | Stimulates the expression of inducible nitric oxide synthase (iNOS) and enhancing NO production | Reactive oxygen species | |||||||||||||||||||||||||||||
| EGFR (epidermal growth factor receptor) family, specifically the tyrosine kinase ErbB-2 (HER2/neu | Inhibit DNA repair mechanism | ||||||||||||||||||||||||||||||
| Stimulation of cyclooxygenase 2 (COX-2) | Nitrosylation of caspase | ||||||||||||||||||||||||||||||
| Increased invasiveness, proliferation, and mobility of cholangiocytes | |||||||||||||||||||||||||||||||
| Prostaglandin E2 production(Rate limitimg step) | |||||||||||||||||||||||||||||||
| Activate cell cycle of cholangiocytes | Inhibit apoptosis of cholangiocytes | Promotes mutagenesis | |||||||||||||||||||||||||||||
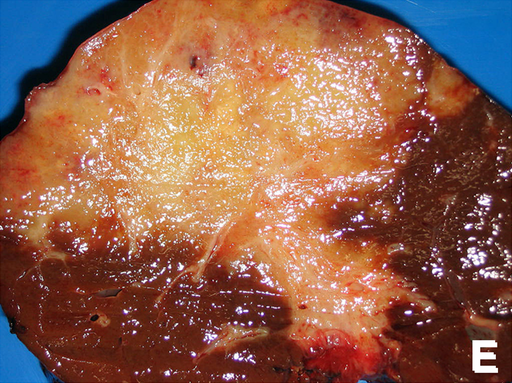
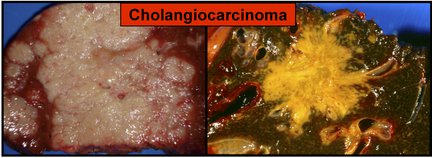
Gross Pathology
Gross pathologic features characteristic to intrahepatic cholangiocarcinoma are divided in three subtypes and include:[8][9][10]
- Mass-forming type
- Nodular lesion or mass in the hepatic parenchyma
- Gray to gray-white, firm and solid carcinoma
- Periductal infiltrating type
- Spreading of the carcinoma along the portal tracts with stricture of the affected bile ducts
- Dilatation of the peripheral bile ducts
- Intraductal growth type
 |
 |
Microscopic Pathology
On microscopic pathology, characteristic findings of cholangiocarcinoma include:
References
- ↑ Fava, G.; Lorenzini, I. (2012). "Molecular Pathogenesis of Cholangiocarcinoma". International Journal of Hepatology. 2012: 1–7. doi:10.1155/2012/630543. ISSN 2090-3448.
- ↑ 2.0 2.1 Sirica A (2005). "Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy". Hepatology. 41 (1): 5–15. PMID 15690474.
- ↑ Holzinger F, Z'graggen K, Büchler M. "Mechanisms of biliary carcinogenesis: a pathogenetic multi-stage cascade towards cholangiocarcinoma". Ann Oncol. 10 Suppl 4: 122–6. PMID 10436802.
- ↑ Gores G (2003). "Cholangiocarcinoma: current concepts and insights". Hepatology. 37 (5): 961–9. PMID 12717374.
- ↑ Wadsworth CA, Dixon PH, Wong JH, Chapman MH, McKay SC, Sharif A, Spalding DR, Pereira SP, Thomas HC, Taylor-Robinson SD, Whittaker J, Williamson C, Khan SA (2011). "Genetic factors in the pathogenesis of cholangiocarcinoma". Dig Dis. 29 (1): 93–7. doi:10.1159/000324688. PMC 3696362. PMID 21691113.
- ↑ Maroni L, Pierantonelli I, Banales JM, Benedetti A, Marzioni M (2013). "The significance of genetics for cholangiocarcinoma development". Ann Transl Med. 1 (3): 28. doi:10.3978/j.issn.2305-5839.2012.10.04. PMC 4200671. PMID 25332972.
- ↑ Kongpetch S, Jusakul A, Ong CK, Lim WK, Rozen SG, Tan P, Teh BT (2015). "Pathogenesis of cholangiocarcinoma: From genetics to signalling pathways". Best Pract Res Clin Gastroenterol. 29 (2): 233–44. doi:10.1016/j.bpg.2015.02.002. PMID 25966424.
- ↑ Nakanuma Y, Sato Y, Harada K, Sasaki M, Xu J, Ikeda H (2010). "Pathological classification of intrahepatic cholangiocarcinoma based on a new concept". World J Hepatol. 2 (12): 419–27. doi:10.4254/wjh.v2.i12.419. PMC 3010511. PMID 21191517.
- ↑ Blechacz B, Komuta M, Roskams T, Gores GJ (2011). "Clinical diagnosis and staging of cholangiocarcinoma". Nat Rev Gastroenterol Hepatol. 8 (9): 512–22. doi:10.1038/nrgastro.2011.131. PMC 3331791. PMID 21808282.
- ↑ Vijgen S, Terris B, Rubbia-Brandt L (2017). "Pathology of intrahepatic cholangiocarcinoma". Hepatobiliary Surg Nutr. 6 (1): 22–34. doi:10.21037/hbsn.2016.11.04. PMC 5332210. PMID 28261592.
- ↑ "File:Cholangiocarcinoma.png - Wikimedia Commons". External link in
|title=(help) - ↑ "File:CCA Cholangiocarcinoma.jpg - Wikimedia Commons".
- ↑ "File:Cholangiocarcinoma - high mag.jpg - Wikimedia Commons".