Zoster vaccine, adjuvanted (Shingrix)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sonya Gelfand
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Zoster vaccine, adjuvanted (Shingrix) is a vaccine that is FDA approved for the prevention of herpes zoster (shingles) in adults aged 50 years and older. Common adverse reactions include pain (78.0%), redness (38.1%), swelling (25.9%), myalgia (44.7%), fatigue (44.5%), headache (37.7%), shivering (26.8%), fever (20.5%), and gastrointestinal symptoms (17.3%).
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Zoster vaccine is a vaccine indicated for prevention of herpes zoster (shingles) in adults aged 50 years and older.
Limitations of Use
- Zoster vaccine is not indicated for prevention of primary varicella infection (chickenpox).
Reconstitution
- Prepare zoster vaccine by reconstituting the lyophilized varicella zoster virus glycoprotein E (gE) antigen component with the accompanying AS01B adjuvant suspension component. The reconstituted vaccine should be an opalescent, colorless to pale brownish liquid. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If either of these conditions exists, the vaccine should not be administered.
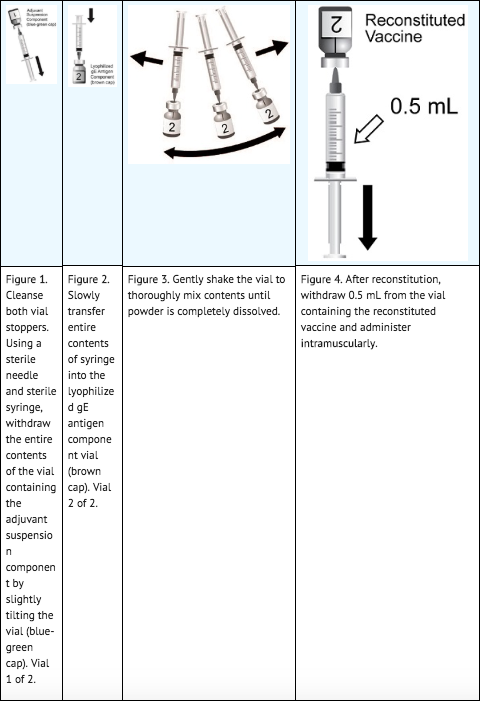
Dose and Schedule
- Two doses (0.5 mL each) administered intramuscularly according to the following schedule: A first dose at Month 0 followed by a second dose administered anytime between 2 and 6 months later.
Dosage Forms and Strengths
- Zoster vaccine is a suspension for injection supplied as a single-dose vial of lyophilized gE antigen component to be reconstituted with the accompanying vial of AS01B adjuvant suspension component. A single dose after reconstitution is 0.5 mL.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding zoster vaccine Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding zoster vaccine Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Zoster vaccine, adjuvanted (Shingrix) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding zoster vaccine Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding zoster vaccine Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Contraindications
- Do not administer zoster vaccine to anyone with a history of a severe allergic reaction (e.g., anaphylaxis) to any component of the vaccine or after a previous dose of zoster vaccine.
Warnings
Preventing and Managing Allergic Vaccine Reactions
- Prior to administration, the healthcare provider should review the immunization history for possible vaccine sensitivity and previous vaccination-related adverse reactions. Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of zoster vaccine.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared with rates in the clinical trials of another vaccine, and may not reflect the rates observed in practice. There is the possibility that broad use of zoster vaccine could reveal adverse reactions not observed in clinical trials.
- Overall, 17,041 adults aged 50 years and older received at least 1 dose of zoster vaccine in 17 clinical studies.
- The safety of zoster vaccine was evaluated by pooling data from 2 placebo-controlled clinical studies (Studies 1 and 2) involving 29,305 subjects aged 50 years and older who received at least one dose of zoster vaccine (n = 14,645) or saline placebo (n = 14,660) administered according to a 0- and 2-month schedule. At the time of vaccination, the mean age of the population was 69 years; 7,286 (24.9%) subjects were aged 50 to 59 years, 4,488 (15.3%) subjects were aged 60 to 69 years, and 17,531 (59.8%) subjects were aged 70 years and older. Both studies were conducted in North America, Latin America, Europe, Asia, and Australia. In the overall population, the majority of subjects were white (74.3%), followed by Asian (18.3%), black (1.4%), and other racial/ethnic groups (6.0%); 58% were female.
Solicited Adverse Events
- In Studies 1 and 2, data on solicited local and general adverse events were collected using standardized diary cards for 7 days following each vaccine dose or placebo (i.e., day of vaccination and the next 6 days) in a subset of subjects (n = 4,886 receiving zoster vaccine, n = 4,881 receiving placebo with at least 1 documented dose). Across both studies, the percentages of subjects aged 50 years and older reporting each solicited local adverse reaction and each solicited general adverse event following administration of zoster vaccine (both doses combined) were pain (78.0%), redness (38.1%), and swelling (25.9%); and myalgia (44.7%), fatigue (44.5%), headache (37.7%), shivering (26.8%), fever (20.5%), and gastrointestinal symptoms (17.3%), respectively.
- The reported frequencies of specific solicited local adverse reactions and general adverse events (overall per subject), by age group, from the 2 studies are presented in Table 1.

- The incidence of solicited local and general symptoms was lower in subjects aged 70 years and older compared with those aged 50 to 69 years.
- The majority of solicited local adverse reactions and general adverse events seen with zoster vaccine had a median duration of 2 to 3 days.
- There were no differences in the proportions of subjects reporting any or grade 3 solicited local reactions between Dose 1 and Dose 2. Headache and shivering were reported more frequently by subjects after Dose 2 (28.2% and 21.4%, respectively) compared with Dose 1 (24.4% and 13.8%, respectively). Grade 3 solicited general adverse events (headache, shivering, myalgia, and fatigue) were reported more frequently by subjects after Dose 2 (2.3%, 3.1%, 3.6%, and 3.5%, respectively) compared with Dose 1 (1.4%, 1.4%, 2.3%, and 2.4%, respectively).
Unsolicited Adverse Events
- Unsolicited adverse events that occurred within 30 days following each vaccination (Day 0 to 29) were recorded on a diary card by all subjects. In the 2 studies, unsolicited adverse events occurring within 30 days of vaccination were reported in 50.5% and 32.0% of subjects who received zoster vaccine (n = 14,645) and placebo (n = 14,660), respectively (Total Vaccinated Cohort). Unsolicited adverse events that occurred in ≥1% of recipients of zoster vaccine and at a rate at least 1.5-fold higher than placebo included chills (3.5% versus 0.2%), injection site pruritus (2.2% versus 0.2%), malaise (1.7% versus 0.3%), arthralgia (1.7% versus 1.2%), nausea (1.4% versus 0.5%), and dizziness (1.2% versus 0.8%).
- Gout (including gouty arthritis) was reported by 0.18% (n = 27) versus 0.05% (n = 8) of subjects who received zoster vaccine and placebo, respectively, within 30 days of vaccination; available information is insufficient to determine a causal relationship with zoster vaccine.
Serious Adverse Events (SAEs)
- In the 2 studies, SAEs were reported at similar rates in subjects who received zoster vaccine (2.3%) and placebo (2.2%) from the first administered dose up to 30 days post last vaccination. SAEs were reported for 10.1% of subjects who received zoster vaccine and for 10.4% of subjects who received placebo from the first administered dose up to 1 year post last vaccination. One subject (<0.01%) reported lymphadenitis and 1 subject (<0.01%) reported fever greater than 39°C; there was a basis for a causal relationship with zoster vaccine.
- Optic ischemic neuropathy was reported in 3 subjects (0.02%) who received zoster vaccine (all within 50 days after vaccination) and 0 subjects who received placebo; available information is insufficient to determine a causal relationship with zoster vaccine.
Deaths
- From the first administered dose up to 30 days post last vaccination, deaths were reported for 0.04% of subjects who received zoster vaccine and 0.05% of subjects who received placebo in the 2 studies. From the first administered dose up to 1 year post last vaccination, deaths were reported for 0.8% of subjects who received zoster vaccine and for 0.9% of subjects who received placebo. Causes of death among subjects were consistent with those generally reported in adult and elderly populations.
Potential Immune-Mediated Diseases
- In the 2 studies, new onset potential immune-mediated diseases (pIMDs) or exacerbation of existing pIMDs were reported for 0.6% of subjects who received zoster vaccine and 0.7% of subjects who received placebo from the first administered dose up to 1 year post last vaccination. The most frequently reported pIMDs occurred with comparable frequencies in the group receiving zoster vaccine and the placebo group.
Dosing Schedule
- In an open-label clinical study, 238 subjects 50 years and older received zoster vaccine as a 0- and 2-month or 0- and 6-month schedule. The safety profile of zoster vaccine was similar when administered according to a 0- and 2-month or 0- and 6-month schedule, and was consistent with that observed in Studies 1 and 2.
Postmarketing Experience
There is limited information regarding Zoster vaccine, adjuvanted (Shingrix) Postmarketing Experience in the drug label.
Drug Interactions
- Concomitant Vaccine Administration
- Immunosuppressive Therapies
Concomitant Vaccine Administration
- For concomitant administration of zoster vaccine with inactivated influenza vaccine.
Immunosuppressive Therapies
- Immunosuppressive therapies may reduce the effectiveness of zoster vaccine.
Use in Specific Populations
Pregnancy
Risk Summary
- All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively. There are no available human data to establish whether there is vaccine-associated risk with zoster vaccine in pregnant women.
- A reproductive and developmental toxicity study was performed in female rats administered zoster vaccine or the AS01B adjuvant alone prior to mating, during gestation, and during lactation. The total dose was 0.2 mL on each occasion (a single human dose of zoster vaccine is 0.5 mL). This study revealed no adverse effects on fetal or pre-weaning development due to zoster vaccine.
Data (Animal)
- In a reproductive and developmental toxicity study, female rats were administered zoster vaccine or the AS01B adjuvant alone by intramuscular injection 28 and 14 days prior to mating, on gestation Days 3, 8, 11, and 15, and on lactation Day 7. The total dose was 0.2 mL on each occasion (a single human dose of zoster vaccine is 0.5 mL). No adverse effects on pre-weaning development up to post-natal Day 25 were observed. There were no vaccine-related fetal malformations or variations.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Zoster vaccine, adjuvanted (Shingrix) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Zoster vaccine, adjuvanted (Shingrix) during labor and delivery.
Nursing Mothers
Risk Summary
- It is not known whether zoster vaccine is excreted in human milk. Data are not available to assess the effects of zoster vaccine on the breastfed infant or on milk production/excretion.
- The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for zoster vaccine and any potential adverse effects on the breastfed child from zoster vaccine or from the underlying maternal condition. For preventive vaccines, the underlying maternal condition is susceptibility to disease prevented by the vaccine.
Pediatric Use
- Safety and effectiveness in individuals younger than 18 years have not been established. Zoster vaccine is not indicated for prevention of primary varicella infection (chickenpox).
Geriatic Use
- Of the total number of subjects who received at least 1 dose of zoster vaccine in the 2 efficacy trials (n = 14,645), 2,243 (15.3%) were aged 60 to 69 years, 6,837 (46.7%) were aged 70 to 79 years, and 1,921 (13.1%) were 80 years and older. There were no clinically meaningful differences in efficacy across the age groups or between these subjects and younger subjects.
- The frequencies of solicited local and general adverse events in subjects aged 70 years and older were lower than in younger adults (aged 50 through 69 years).
Gender
There is no FDA guidance on the use of Zoster vaccine, adjuvanted (Shingrix) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Zoster vaccine, adjuvanted (Shingrix) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Zoster vaccine, adjuvanted (Shingrix) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Zoster vaccine, adjuvanted (Shingrix) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Zoster vaccine, adjuvanted (Shingrix) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Zoster vaccine, adjuvanted (Shingrix) in patients who are immunocompromised.
Administration and Monitoring
Administration
- For intramuscular injection only.
- After reconstitution, administer zoster vaccine immediately or store refrigerated between 2° and 8°C (36° and 46°F) and use within 6 hours. Discard reconstituted vaccine if not used within 6 hours.
- Use a separate sterile needle and sterile syringe for each individual. The preferred site for intramuscular injection is the deltoid region of the upper arm.
Monitoring
There is limited information regarding Zoster vaccine, adjuvanted (Shingrix) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Zoster vaccine, adjuvanted (Shingrix) and IV administrations.
Overdosage
There is limited information regarding Zoster vaccine, adjuvanted (Shingrix) overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
| Vaccine description | |
|---|---|
| Target disease | Herpes zoster, postherpetic neuralgia, Ramsay Hunt syndrome type II, chickenpox |
| Clinical data | |
| Trade names | Zostavax, Shingrix |
| [[Regulation of therapeutic goods |Template:Engvar data]] | |
| Pregnancy category |
|
| Routes of administration | subcutaneous injection (Zostavax), intramuscular injection (Shingrix) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| ChemSpider |
|
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| (verify) | |
Mechanism of Action
- The risk of developing herpes zoster (HZ) increases with age and appears to be related to a decline in VZV-specific immunity. Zoster vaccine was shown to boost VZV-specific immune response, which is thought to be the mechanism by which it protects against zoster disease.
Structure
There is limited information regarding Zoster vaccine, adjuvanted (Shingrix) Structure in the drug label.
Pharmacodynamics
There is limited information regarding Zoster vaccine, adjuvanted (Shingrix) Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Zoster vaccine, adjuvanted (Shingrix) Pharmacokinetics in the drug label.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Zoster vaccine has not been evaluated for its carcinogenic or mutagenic potential. Vaccination of female rats with zoster vaccine had no effect on fertility. In a male fertility study, rats were vaccinated with 0.1 mL of zoster vaccine (a single human dose is 0.5 mL) on 42, 28, and 14 days prior to mating. There were no effects on male fertility.
Clinical Studies
Efficacy in Subjects 50 Years and Older
- Study 1 was a randomized, placebo-controlled, observer-blind clinical study conducted in 18 countries. Randomization was stratified (8:5:3:1) by age: 50 to 59 years, 60 to 69 years, 70 to 79 years, and ≥80 years. The study excluded, among others, subjects who were immunocompromised, had a history of previous HZ, were vaccinated against varicella or HZ, and patients whose survival was not expected to be at least 4 years or with conditions that might interfere with study evaluations. Subjects were followed for the development of HZ and postherpetic neuralgia (PHN) for a median of 3.1 years (range: 0 to 3.7 years). Suspected HZ cases were followed prospectively for the development of PHN, an HZ-related complication defined as HZ-associated pain (rated as 3 or greater on a 0- to 10-point scale by the study subject) occurring or persisting at least 90 days following the onset of rash in confirmed cases of HZ.
- The primary efficacy analysis population (referred to as the modified Total Vaccinated Cohort [mTVC]) included 14,759 subjects aged 50 years and older who received 2 doses (0 and 2 months) of either zoster vaccine (n = 7,344) or placebo (n = 7,415) and did not develop a confirmed case of HZ within 1 month after the second dose. In the mTVC population, 61.2% were female; 72.3% were white, 18.9% were Asian, 1.7% were black, and 7.0% were of other racial/ethnic groups. The mean age of subjects was 62.3 years.
- Confirmed HZ cases were determined by either Polymerase Chain Reaction (PCR) (89.4%) or by a Clinical Evaluation Committee (10.6%).
Efficacy against Herpes Zoster
- Compared with placebo, zoster vaccine significantly reduced the risk of developing HZ by 97.2% (95% CI: 93.7, 99.0) in subjects 50 years and older (Table 2).

- In a descriptive analysis, vaccine efficacy against HZ in subjects aged 50 years and older was 93.1% (95% CI: 81.3, 98.2) in the fourth year post-vaccination.
Occurrence of PHN
- Among all subjects aged 50 years or older in the mTVC, no cases of PHN were reported in the vaccine group compared with 18 cases reported in the placebo group.
Efficacy in Subjects 70 Years and Older
- Study 2 was a randomized, placebo-controlled, observer-blind clinical study conducted in 18 countries. Randomization was stratified (3:1) by age: 70 to 79 years and ≥80 years. With the exception of age, the study exclusion criteria were the same as for Study 1. Subjects were followed for the development of HZ and PHN for a median of 3.9 years (range: 0 to 4.5 years). Suspected HZ cases were followed prospectively for the development of PHN as for Study 1.
- The primary efficacy analysis population (mTVC) included 13,163 subjects aged 70 years and older who received 2 doses (0 and 2 months) of either zoster vaccine (n = 6,541) or placebo (n = 6,622) and did not develop a confirmed case of HZ within 1 month after the second dose. In the mTVC population, 54.7% were female; 77.6% were white, 17.1% were Asian, 1.0% were black, and 4.2% were of other racial/ethnic groups. The mean age of subjects was 75.5 years.
- Confirmed HZ cases were determined by either PCR (92.3%) or by a Clinical Evaluation Committee (7.7%).
Efficacy against Herpes Zoster
- Vaccine efficacy results against HZ in subjects 70 years and older are shown in Table 3.

- In a descriptive analysis, vaccine efficacy against HZ in subjects 70 years and older was 85.1% (95% CI: 64.5, 94.8) in the fourth year after vaccination.
Efficacy against PHN
- Among all subjects aged 70 years or older in the mTVC, 4 cases of PHN were reported in the vaccine group, compared with 28 cases reported in the placebo group. Vaccine efficacy against PHN was 85.5% (95% CI: [58.5; 96.3]). The benefit of zoster vaccine in the prevention of PHN can be attributed to the effect of the vaccine on the prevention of HZ.
Reduction of Use of Pain Medication
- Among subjects with confirmed HZ, the use of HZ-associated pain medications was reported for 10 of 23 subjects (43.5%) who received zoster vaccine and for 160 of 223 subjects (71.7%) who received placebo.
Pooled Efficacy Analyses across Studies 1 and 2
- The efficacy of zoster vaccine to prevent HZ and PHN in subjects 70 years and older was evaluated by combining the results from Studies 1 and 2 through a pre-specified pooled analysis in the mTVC. A total of 8,250 and 8,346 subjects who received zoster vaccine and placebo, respectively, were included in the pooled mTVC analysis.
Efficacy against Herpes Zoster
- Compared with placebo, zoster vaccine significantly reduced the risk of developing HZ by 91.3% (95% CI: 86.9, 94.5) in subjects 70 years and older (Table 4).

Efficacy against PHN
- Table 5 compares the overall rates of PHN in the vaccine and placebo groups across both studies.

- The benefit of zoster vaccine in the prevention of PHN can be attributed to the effect of the vaccine on the prevention of HZ. The efficacy of zoster vaccine in the prevention of PHN in subjects with confirmed HZ could not be demonstrated.
Immunological Evaluation to Support Dosing Schedule
- A measure of the immune response that confers protection against HZ is unknown. Anti-gE antibody levels were measured by anti-gE enzyme-linked immunosorbent assay (gE ELISA) and were used to support the dosing schedule.
- In an open-label clinical study, 238 subjects 50 years and older received zoster vaccine on either a 0- and 2-month or 0- and 6-month schedule. Non-inferiority of the 0- and 6-month schedule compared with the 0- and 2-month schedule based on anti-gE ELISA GMCs 1 month after the second dose was demonstrated.
Concomitant Administration with Influenza Vaccine
- In an open-label clinical study, subjects 50 years and older received 1 dose each of zoster vaccine and Fluarix Quadrivalent (QIV) at Month 0 and 1 dose of zoster vaccine at Month 2 (n = 413), or 1 dose of QIV at Month 0 and 1 dose of zoster vaccine at Months 2 and 4 (n = 415). There was no evidence for interference in the immune response to any of the antigens contained in zoster vaccine or the coadministered vaccine.
How Supplied
- Zoster vaccine is supplied as 2 components: A single-dose vial of lyophilized gE antigen component (brown cap) and a single-dose vial of adjuvant suspension component (blue-green cap) (packaged without syringes or needles).
- An outer package of 1 dose (NDC 58160-819-12) contains:
- Adjuvant Suspension Component (Vial 1 of 2) NDC 58160-829-01
- Lyophilized gE Antigen Component (Vial 2 of 2) NDC 58160-828-01
- An outer package of 10 doses (NDC 58160-823-11) contains:
- Adjuvant Suspension Component (10 vials) NDC 58160-829-03
- Lyophilized gE Antigen Component (10 vials) NDC 58160-828-03
Storage
Storage before Reconstitution
- Adjuvant suspension component vials: Store refrigerated between 2° and 8°C (36° and 46°F). Protect vials from light. Do not freeze. Discard if the adjuvant suspension has been frozen.
- Lyophilized gE antigen component vials: Store refrigerated between 2° and 8°C (36° and 46°F). Protect vials from light. Do not freeze. Discard if the antigen component has been frozen.
Storage after Reconstitution
- After reconstitution, administer zoster vaccine immediately or store refrigerated between 2° and 8°C (36° and 46°F) and use within 6 hours. Discard reconstituted vaccine if not used within 6 hours. Do not freeze. Discard if the vaccine has been frozen.
Images
Drug Images
{{#ask: Page Name::Zoster vaccine, adjuvanted (Shingrix) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel






{{#ask: Label Page::Zoster vaccine, adjuvanted (Shingrix) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Inform patients of the potential benefits and risks of immunization with zoster vaccine and of the importance of completing the 2-dose immunization series according to the schedule.
- Inform patients about the potential for adverse reactions that have been temporally associated with administration of zoster vaccine.
Precautions with Alcohol
Alcohol-Zoster vaccine, adjuvanted (Shingrix) interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Shingrix
Look-Alike Drug Names
There is limited information regarding Zoster vaccine, adjuvanted (Shingrix) Look-Alike Drug Names in the drug label.
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- Pages with script errors
- Drugs with non-standard legal status
- Chemicals that do not have a ChemSpider ID assigned
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drug has EMA link
- Drugs that are a vaccine