X-linked agammaglobulinemia pathophysiology
|
X-linked agammaglobulinemia Microchapters |
|
Differentiating X-linked agammaglobulinemia from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
X-linked agammaglobulinemia pathophysiology On the Web |
|
American Roentgen Ray Society Images of X-linked agammaglobulinemia pathophysiology |
|
Directions to Hospitals Treating X-linked agammaglobulinemia |
|
Risk calculators and risk factors for X-linked agammaglobulinemia pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Pathophysiology
XLA patients are specifically susceptible to viruses of the Enterovirus family, and mostly to: polio virus, coxsackie virus (hand, foot, and mouth disease) and Echoviruses. These may cause severe central nervous system conditions as chronic encephalitis, meningitis and death. An experimental anti-viral agent, pleconaril, is active against picornaviruses. XLA patients, however, are apparently immune to the Epstein-Barr virus (EBV), as they lack B cells needed for the viral infection.
It is not known if XLA patients are able to generate an allergic reaction, as they lack functional IgE antibodies.
Genetics
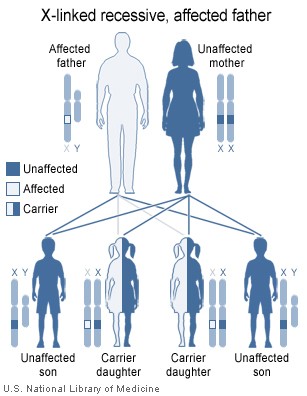
The gene Bruton's tyrosine kinase (Btk) plays an essential role in the maturation B cells in the bone marrow, and when mutated, immature pre-B lymphocytes are unable to develop into mature B cells that leave the bone marrow into the blood stream. The disorder is X-linked (it is on the X chromosome), and is almost entirely limited to the sons of asymptomatic female carriers .[1] This is because males have only one copy of the X chromosome, while females have two copies; one normal copy of an X chromosome can compensate in for mutations in the other X chromosome. Females carriers have a 50% chance of giving birth to a male child with XLA.
An XLA patient will pass on the gene, and all of his daughters will be XLA carriers, meaning that any male grandchildren from an XLA patient's daughters have a 50% chance of inheriting XLA. A female XLA patient can only arise as the child of an XLA patient and a carrier mother. XLA can also rarely result from a spontaneous mutation in the fetus of a non-carrier mother.

|
|
References
- ↑ X-Linked Agammaglobulinemia Patient and Family Handbook for The Primary Immune Diseases. Third Edition. 2001. Published by the Immune Deficiency Foundation