Valproic acid capsule/solution description
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Description
Depakene (valproic acid) is a carboxylic acid designated as 2-propylpentanoic acid. It is also known as dipropylacetic acid. Valproic acid has the following structure:
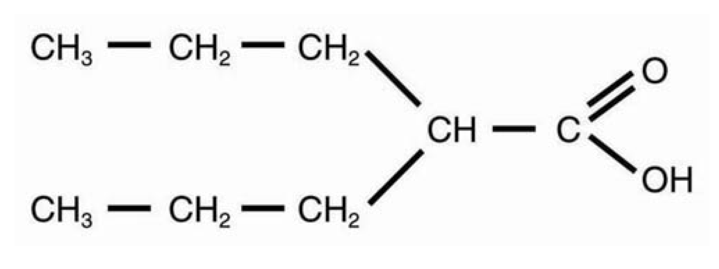 |
Valproic acid (pKa 4.8) has a molecular weight of 144 and occurs as a colorless liquid with a characteristic odor. It is slightly soluble in water (1.3 mg/mL) and very soluble in organic solvents.
Depakene capsules and syrup are antiepileptics for oral administration. Each soft elastic capsule contains 250 mg valproic acid. The syrup contains the equivalent of 250 mg valproic acid per 5 mL as the sodium salt.
Inactive Ingredients
250 mg capsules: corn oil, FD&C Yellow No. 6, gelatin, glycerin, iron oxide, methylparaben, propylparaben, and titanium dioxide.
Oral Solution: FD&C Red No. 40, glycerin, methylparaben, propylparaben, sorbitol, sucrose, water, and natural and artificial flavors.[1]
References
Adapted from the FDA Package Insert.