Valganciclovir hydrochloride clinical studies
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Clinical Studies
Adult Patients
Induction Therapy of CMV Retinitis
In one randomized open-label controlled study, 160 patients with AIDS and newly diagnosed CMV retinitis were randomized to receive treatment with either Valcyte tablets (900 mg twice daily for 21 days, then 900 mg once daily for 7 days) or with intravenous ganciclovir solution (5 mg/kg twice daily for 21 days, then 5 mg/kg once daily for 7 days). Study participants were: male (91%), White (53%), Hispanic (31%), and Black (11%). The median age was 39 years, the median baseline HIV-1 RNA was 4.9 log10, and the median CD4 cell count was 23 cells/mm3. A determination of CMV retinitis progression by the masked review of retinal photographs taken at baseline and Week 4 was the primary outcome measurement of the 3-week induction therapy. Table 17 provides the outcomes at 4 weeks.
 |
Maintenance Therapy of CMV Retinitis
No comparative clinical data are available on the efficacy of Valcyte tablets for the maintenance therapy of CMV retinitis because all patients in the CMV retinitis study received open-label Valcyte tablets after Week 4. However, the AUC for ganciclovir is similar following administration of 900 mg Valcyte tablets once daily and 5 mg/kg intravenous ganciclovir once daily. Although the ganciclovir Cmax is lower following Valcyte tablets administration compared to intravenous ganciclovir, it is higher than the Cmax obtained following oral ganciclovir administration [see Figure 1 in Clinical Pharmacology (12.3)]. Therefore, use of Valcyte tablets as maintenance therapy is supported by a plasma concentration-time profile similar to that of two approved products for maintenance therapy of CMV retinitis.
Prevention of CMV Disease in Heart, Kidney, Kidney-Pancreas, or Liver Transplantation
A double blind, double-dummy active comparator study was conducted in 372 heart, liver, kidney, or kidney-pancreas transplant patients at high risk for CMV disease (D+/R-). Patients were randomized (2 Valcyte: 1 oral ganciclovir) to receive either Valcyte tablets (900 mg once daily) or oral ganciclovir (1000 mg three times a day) starting within 10 days of transplantation until Day 100 post-transplant. The proportion of patients who developed CMV disease, including CMV syndrome and/or tissue-invasive disease during the first 6 months post-transplant was similar between the Valcyte tablets arm (12.1%, N=239) and the oral ganciclovir arm (15.2%, N=125). However, in liver transplant patients, the incidence of tissue-invasive CMV disease was significantly higher in the Valcyte group compared with the ganciclovir group. These results are summarized in Table 18.
Mortality at six months was 3.7% (9/244) in the Valcyte group and 1.6% (2/126) in the oral ganciclovir group.
 |
Prevention of CMV Disease in Kidney Transplantation
A double-blind, placebo-controlled study was conducted in 326 kidney transplant patients at high risk for CMV disease (D+/R-) to assess the efficacy and safety of extending Valcyte CMV prophylaxis from 100 to 200 days post-transplant. Patients were randomized (1:1) to receive Valcyte tablets (900 mg once daily) within 10 days of transplantation either until Day 200 post-transplant or until Day 100 post-transplant followed by 100 days of placebo. Extending CMV prophylaxis with Valcyte until Day 200 post-transplant demonstrated superiority in preventing CMV disease within the first 12 months post-transplant in high risk kidney transplant patients compared to the 100 day dosing regimen (primary endpoint). These results are summarized in Table 19.
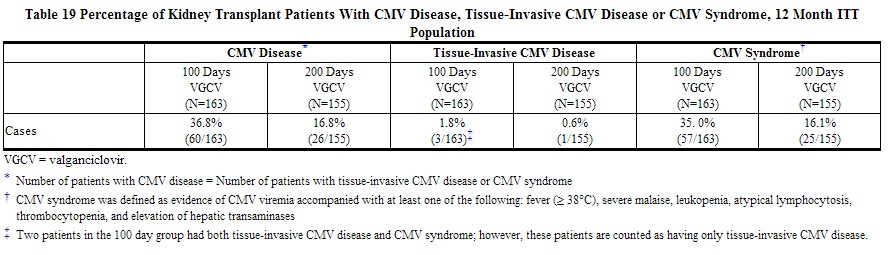 |
The percentage of kidney transplant patients with CMV disease at 24 months post-transplant was 38.7% (63/163) for the 100 day dosing regimen and 21.3% (33/155) for the 200 day dosing regimen.
Pediatric Patients
Prevention of CMV in Pediatric Solid Organ Transplant Recipients
Sixty-three children, 4 months to 16 years of age, who had a solid organ transplant (kidney 33, liver 17, heart 12, and kidney/liver 1) and were at risk for developing CMV disease, were enrolled in an open-label, safety, and pharmacokinetic study of oral Valcyte (Valcyte for oral solution or tablets). Patients received Valcyte once daily as soon as possible after transplant until a maximum of 100 days post-transplant. The daily doses of Valcyte were calculated at each study visit based on body surface area and a modified creatinine clearance [see Dosage and Administration (2.3)].
The pharmacokinetics of ganciclovir were similar across organ transplant types and age ranges. The mean daily ganciclovir exposures in pediatric patients were comparable to those observed in adult solid organ transplant patients receiving Valcyte 900 mg once daily [see Clinical Pharmacology (12.3)]. No case of CMV disease was reported during the study. CMV viremia was reported in 7 (11%) patients during the study; however, none of these events fulfilled the definition of CMV syndrome. Based on the pharmacokinetic, safety, and efficacy data from this study and extrapolated efficacy data from the adult study, oral Valcyte is indicated for the prevention of CMV disease in kidney and heart transplant children 4 months to 16 years of age at risk for developing CMV disease. Valcyte is not approved in adults for CMV prophylaxis in liver transplant patients; therefore, Valcyte is not recommended for CMV prophylaxis in pediatric liver transplant patients because efficacy cannot be extrapolated from adults.[1]
References
- ↑ "http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021304s008,022257s003lbl.pdf" (PDF). External link in
|title=(help)
Adapted from the FDA Package Insert.