Tobramycin clinical studies
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Clinical Studies
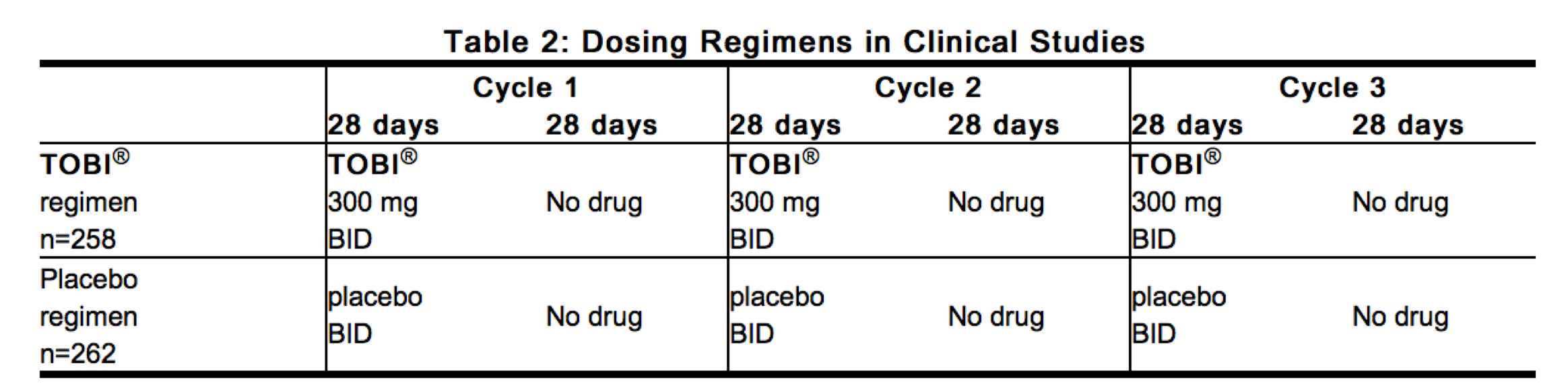
Two identically designed, double-blind, randomized, placebo-controlled, parallel group, 24-week clinical studies (Study 1 and Study 2) at a total of 69 cystic fibrosis centers in the United States were conducted in cystic fibrosis patients with P. aeruginosa. Subjects who were less than 6 years of age, had a baseline creatinine of >2 mg/dL, or had Burkholderia cepacia isolated from sputum were excluded. All subjects had baseline FEV1 % predicted between 25% and 75%. In these clinical studies, 258 patients received TOBI® therapy on an outpatient basis (see Table 2) using a hand-held PARI LC PLUS™ Reusable Nebulizer with a DeVilbiss® Pulmo-Aide® compressor.
 |
All patients received either TOBI® or placebo (saline with 1.25 mg quinine for flavoring) in addition to standard treatment recommended for cystic fibrosis patients, which included oral and parenteral anti-pseudomonal therapy, β2-agonists, cromolyn, inhaled steroids, and airway clearance techniques. In addition, approximately 77% of patients were concurrently treated with dornase alfa (PULMOZYME®, Genentech).
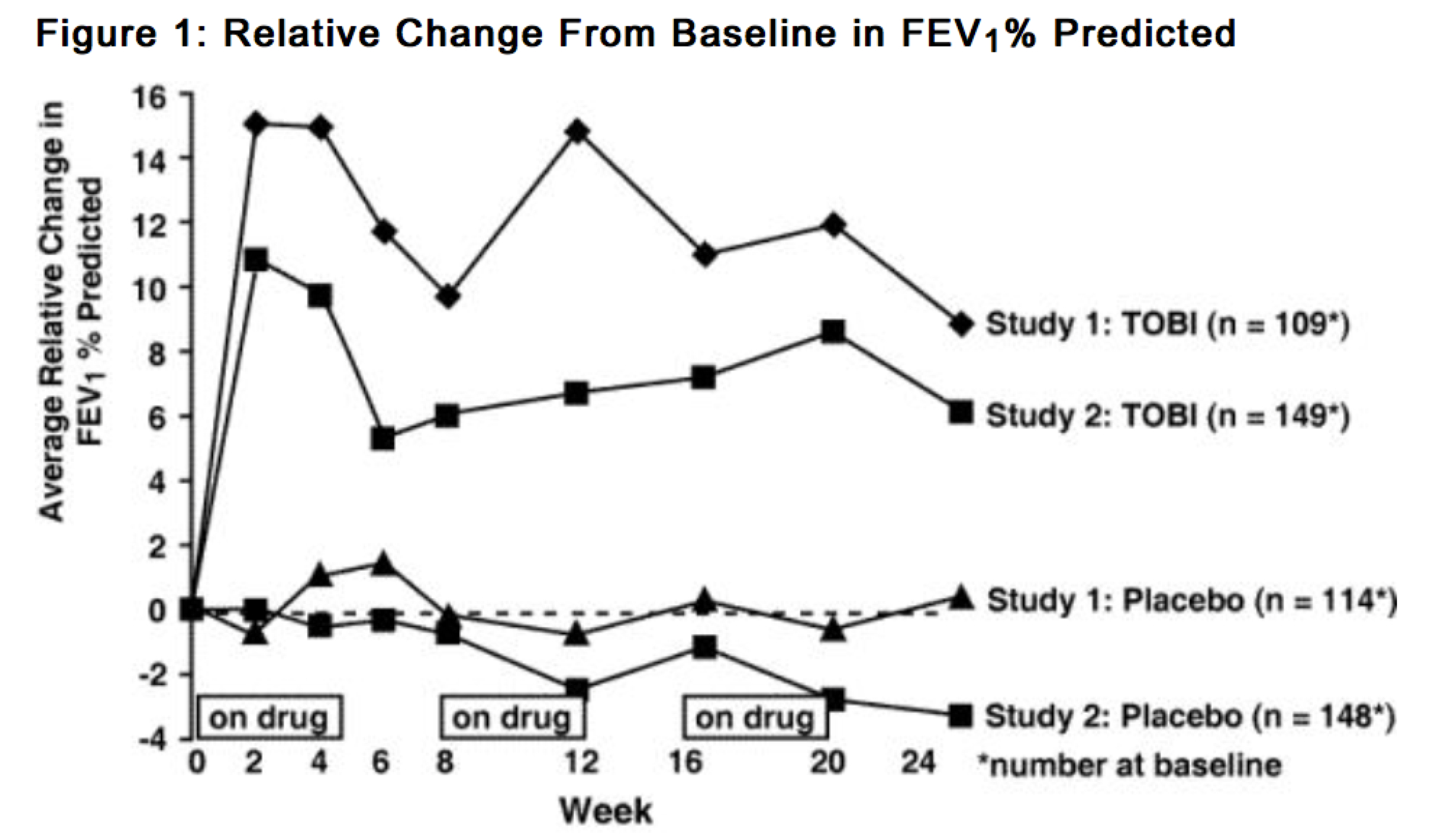
In each study, TOBI®-treated patients experienced significant improvement in pulmonary function. Improvement was demonstrated in the TOBI® group in Study 1 by an average increase in FEV1 % predicted of about 11% relative to baseline (Week 0) during 24 weeks compared to no average change in placebo patients. In Study 2, TOBI®-treated patients had an average increase of about 7% compared to an average decrease of about 1% in placebo patients. Figure 1 shows the average relative change in FEV1% predicted over 24 weeks for both studies.
 |
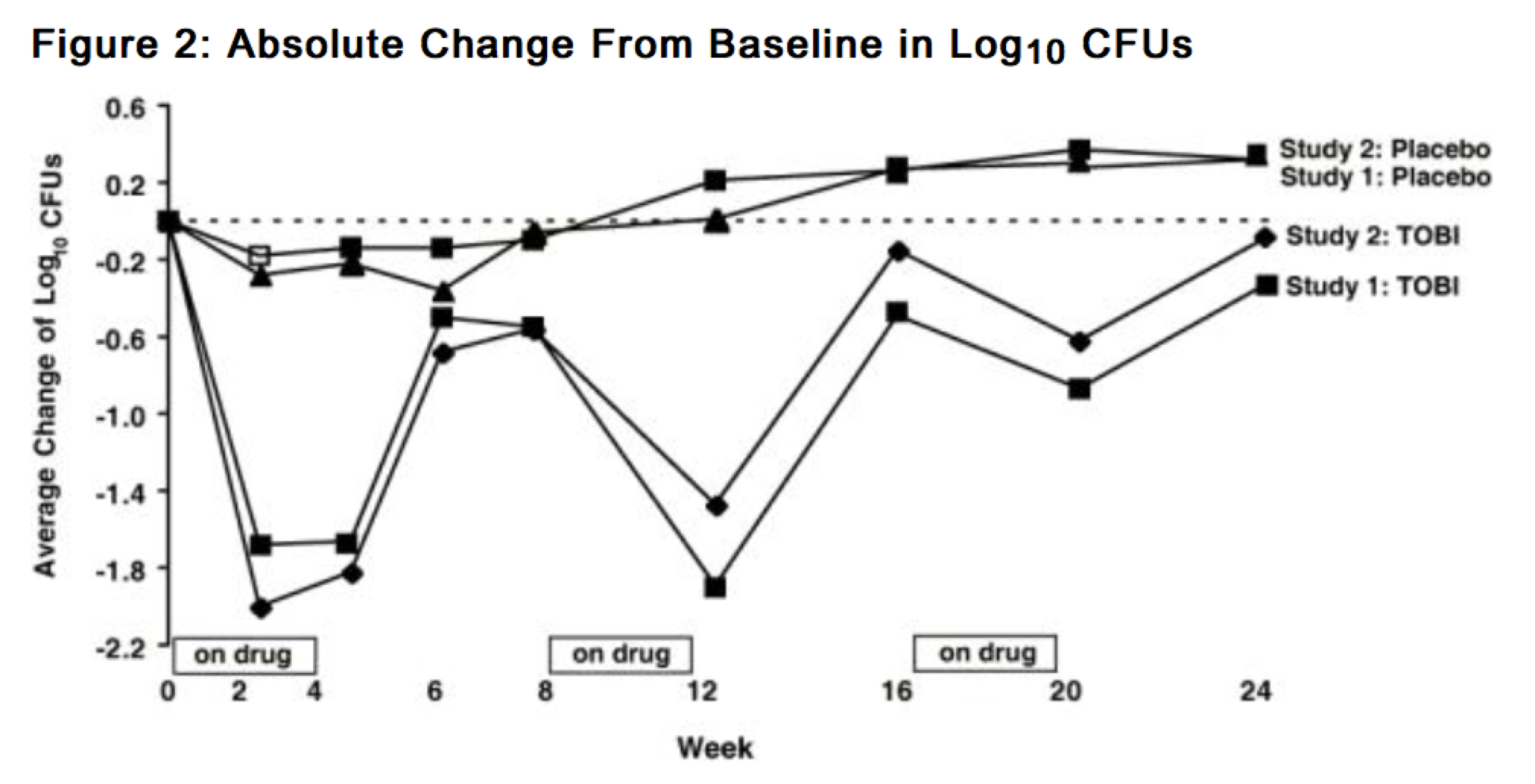
In each study, TOBI® therapy resulted in a significant reduction in the number of P. aeruginosa colony forming units (CFUs) in sputum during the on-drug periods. Sputum bacterial density returned to baseline during the off-drug periods. Reductions in sputum bacterial density were smaller in each successive cycle. (see Figure 2).
 |
Patients treated with TOBI® were hospitalized for an average of 5.1 days compared to 8.1 days for placebo patients. Patients treated with TOBI® required an average of 9.6 days of parenteral anti-pseudomonal antibiotic treatment compared to 14.1 days for placebo patients. During the 6 months of treatment, 40% of TOBI® patients and 53% of placebo patients were treated with parenteral anti-pseudomonal antibiotics.
The relationship between in-vitro susceptibility test results and clinical outcome with TOBI® therapy is not clear. However, 4 TOBI® patients who began the clinical trial with P. aeruginosa isolates having MIC values ≥128 µg/mL did not experience an improvement in FEV1 or a decrease in sputum bacterial density.
Treatment with TOBI® did not affect the susceptibility of the majority of P. aeruginosa isolates during the 6-month studies. However, some P. aeruginosa isolates did exhibit increased tobramycin MICs. The percentage of patients with P. aeruginosa isolates with tobramycin MICs ≥16 µg/mL was 13% at the beginning, and 23% at the end of 6 months of the TOBI® regimen.[1]
References
Adapted from the FDA Package Insert.