Tbo-filgrastim
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Aparna Vuppala, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Tbo-filgrastim is an colony stimulating factor that is FDA approved for the treatment of to reduce the duration of severe neutropenia in patients with non‑myeloid malignancies receiving myelosuppressive anti‑cancer drugs associated with a clinically significant incidence of febrile neutropenia. Common adverse reactions include Splenic rupture , Acute Respiratory Distress Syndrome , Serious Allergic Reactions , Capillary leak syndrome.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Non‑myeloid malignancies
- Tbo-filgrastim is indicated to reduce the duration of severe neutropenia in patients with non‑myeloid malignancies receiving myelosuppressive anti‑cancer drugs associated with a clinically significant incidence of febrile neutropenia.
Dosing Information
- The recommended dose of Tbo-filgrastim is 5 mcg/kg per day administered as a subcutaneous injection. Administer the first dose of Tbo-filgrastim no earlier than 24 hours following myelosuppressive chemotherapy. Do not administer Tbo-filgrastim within 24 hours prior to chemotherapy .
- Daily dosing with Tbo-filgrastim should continue until the expected neutrophil nadir is passed and the neutrophil count has recovered to the normal range. Monitor complete blood count (CBC) prior to chemotherapy and twice per week until recovery.
General Considerations for Administration
- Tbo-filgrastim may be administered by either a healthcare professional or by a patient or caregiver. Before a decision is made to allow Tbo-filgrastim to be administered by a patient or caregiver, ensure that the patient is an appropriate candidate for self-administration or administration by a caregiver. Proper training on storage, preparation, and administration technique should be provided. If a patient or caregiver is not an appropriate candidate for any reason, then in such patients, Tbo-filgrastim should be administered by a healthcare professional.
- Dispense only the pre-filled syringe without a safety needle guard device to patient or caregiver. Instruct patients and caregivers to follow the Instructions for Use provided with the Tbo-filgrastim pre-filled syringe to properly administer an injection after training by a healthcare professional.
- Visually inspect parenteral drug products for particulate matter and discoloration prior to administration.
- Do not administer Tbo-filgrastim if discoloration or particulates are observed.
- The prefilled syringe is for single use only. Discard unused portions.
- Recommended sites for subcutaneous Tbo-filgrastim injections include the abdomen (except for the two-inch area around the navel), the front of the middle thighs, the upper outer areas of the buttocks, or the upper back portion of the upper arms. The injection site should be varied daily. Tbo-filgrastim should not be injected into an area that is tender, red, bruised or hard, or that has scars or stretch marks.
Instructions for Use of the Safety Needle Guard Device by Healthcare Professionals
- Hold the syringe assembly by the open sides of the device and remove the needle shield.

Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Tbo-filgrastim in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Tbo-filgrastim in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Tbo-filgrastim in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Tbo-filgrastim in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Tbo-filgrastim in pediatric patients.
Contraindications
- None.
Warnings
Splenic Rupture
- Splenic rupture, including fatal cases, can occur following administration of human granulocyte colony-stimulating growth factor. In patients who report upper abdominal or shoulder pain after receiving Tbo-filgrastim, discontinue Tbo-filgrastim and evaluate for an enlarged spleen or splenic rupture.
Acute Respiratory Distress Syndrome (ARDS)
- Acute respiratory distress syndrome (ARDS) can occur in patients receiving human granulocyte colony-stimulating growth factor. Evaluate patients who develop fever and lung infiltrates or respiratory distress after receiving Tbo-filgrastim, for ARDS. Discontinue Tbo-filgrastim in patients with ARDS.
Allergic Reactions
- Serious allergic reactions including anaphylaxis can occur in patients receiving human granulocyte colony-stimulating growth factor. Reactions can occur on initial exposure. The administration of antihistamines‚ steroids‚ bronchodilators‚ and/or epinephrine may reduce the severity of the reactions. Permanently discontinue Tbo-filgrastim in patients with serious allergic reactions. Do not administer Tbo-filgrastim to patients with a history of serious allergic reactions to filgrastim or pegfilgrastim.
Use in Patients with Sickle Cell Disease
- Severe and sometimes fatal sickle cell crises can occur in patients with sickle cell disease receiving human granulocyte colony-stimulating growth factor. Consider the potential risks and benefits prior to the administration of human granulocyte colony-stimulating growth factor in patients with sickle cell disease. Discontinue Tbo-filgrastim in patients undergoing a sickle cell crisis.
Capillary Leak Syndrome
- Capillary leak syndrome (CLS) can occur in patients receiving human granulocyte colony-stimulating growth factor and is characterized by hypotension, hypoalbuminemia, edema and hemoconcentration. Episodes vary in frequency, severity and may be life-threatening if treatment is delayed. Patients who develop symptoms of capillary leak syndrome should be closely monitored and receive standard symptomatic treatment, which may include a need for intensive care.
Potential for Tumor Growth Stimulatory Effects on Malignant Cells
- The granulocyte colony-stimulating factor (G‑CSF) receptor through which Tbo-filgrastim acts has been found on tumor cell lines. The possibility that Tbo-filgrastim acts as a growth factor for any tumor type, including myeloid malignancies and myelodysplasia, diseases for which Tbo-filgrastim is not approved, cannot be excluded.
Adverse Reactions
Clinical Trials Experience
- The following potential serious adverse reactions are discussed in greater detail in other sections of the labeling:
- Splenic Rupture
- Acute Respiratory Distress Syndrome
- Serious Allergic Reactions
- Use in Patients with Sickle Cell Disease
- Capillary leak Syndrome
- Potential for Tumor Growth Stimulatory Effects on Malignant Cells
- The most common treatment-emergent adverse reaction that occurred at an incidence of at least 1% or greater in patients treated with Tbo-filgrastim at the recommended dose and was numerically two times more frequent than in the placebo group was bone pain.
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
- Tbo-filgrastim clinical trials safety data are based upon the results of three randomized clinical trials in patients receiving myeloablative chemotherapy for breast cancer (N=348), lung cancer (N=240) and non-Hodgkin’s lymphoma (N=92). In the breast cancer study, 99% of patients were female, the median age was 50 years, and 86% of patients were Caucasian. In the lung cancer study, 80% of patients were male, the median age was 58 years, and 95% of patients were Caucasian. In the non-Hodgkin’s lymphoma study, 52% of patients were male, the median age was 55 years, and 88% of patients were Caucasian. In all three studies a placebo (Cycle 1 of the breast cancer study only) or a non-US-approved filgrastim product were used as controls. Both Tbo-filgrastim and the non-US-approved filgrastim product were administered at 5 mcg/kg subcutaneously once daily beginning one day after chemotherapy for at least five days and continued to a maximum of 14 days or until an ANC of ≥10,000 x 106/L after nadir was reached.
- Bone pain was the most frequent treatment-emergent adverse reaction that occurred in at least 1% or greater in patients treated with Tbo-filgrastim at the recommended dose and was numerically two times more frequent than in the placebo group. Theoverall incidence of bone pain in Cycle 1 of treatment was 3.4% (3.4% Tbo-filgrastim, 1.4% placebo, 7.5% non-US-approved filgrastim product).
Leukocytosis
- In clinical studies, leukocytosis (WBC counts > 100,000 x 106/L) was observed in less than 1% patients with non-myeloid malignancies receiving Tbo-filgrastim. No complications attributable to leukocytosis were reported in clinical studies.
Additional Adverse Reactions
- Other adverse reactions known to occur following administration of granulocyte colony-stimulating factors include myalgia, headache, vomiting, Sweet’s syndrome (acute febrile neutrophilic dermatosis), cutaneous vasculitis and thrombocytopenia.
Immunogenicity
- As with all therapeutic proteins, there is a potential for immunogenicity. The incidence of antibody development in patients receiving Tbo-filgrastim has not been adequately determined.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Tbo-filgrastim in the drug label.
Drug Interactions
- No formal drug interaction studies between Tbo-filgrastim and other drugs have been performed.
- Drugs which may potentiate the release of neutrophils‚ such as lithium‚ should be used with caution.
- Increased hematopoietic activity of the bone marrow in response to growth factor therapy has been associated with transient positive bone imaging changes. This should be considered when interpreting bone-imaging results.
Use in Specific Populations
Pregnancy
Risk Summary
- There are no adequate and well-controlled studies of Tbo-filgrastim in pregnant women. In animal reproduction studies, treatment of pregnant rabbits with tbo-filgrastim resulted in increased spontaneous abortion and fetal malformations at systemic exposures substantially higher than the human exposure. Tbo-filgrastim should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Animal Data
- In an embryofetal developmental study, pregnant rabbits were administered subcutaneous doses of tbo-filgrastim during the period of organogenesis at 1, 10 and 100 mcg/kg/day. Increased abortions were evident in rabbits treated with tbo-filgrastim at 100 mcg/kg/day. This dose was maternally toxic as demonstrated by reduced body weight. Other embryofetal findings at this dose level consisted of post-implantation loss‚ decrease in mean live litter size and fetal weight, and fetal malformations such as malformed hindlimbs and cleft palate. The dose of 100 mcg/kg/day corresponds to a systemic exposure (AUC) of approximately 50-90 times the exposures observed in patients treated with the clinical tbo-filgrastim
- dose of 5 mcg/kg/day.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Tbo-filgrastim in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Tbo-filgrastim during labor and delivery.
Nursing Mothers
- It is not known whether tbo-filgrastim is secreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Tbo-filgrastim is administered to a nursing woman. Other recombinant G-CSF products are poorly secreted in breast milk and G-CSF is not orally absorbed by neonates.
Pediatric Use
- The safety and effectiveness of Tbo-filgrastim in pediatric patients have not been established.
Geriatic Use
- Among 677 cancer patients enrolled in clinical trials of Tbo-filgrastim, a total of 111 patients were 65 years of age and older. No overall differences in safety or effectiveness were observed between patients age 65 and older and younger patients.
Gender
There is no FDA guidance on the use of Tbo-filgrastim with respect to specific gender populations.
Race
There is no FDA guidance on the use of Tbo-filgrastim with respect to specific racial populations.
Renal Impairment
- The safety and efficacy of Tbo-filgrastim have not been studied in patients with moderate or severe renal impairment. No dose adjustment is recommended for patients with mild renal impairment
Hepatic Impairment
- The safety and efficacy of Tbo-filgrastim have not been studied in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Tbo-filgrastim in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Tbo-filgrastim in patients who are immunocompromised.
Administration and Monitoring
Administration
- Subcutaneous
Monitoring
There is limited information regarding Monitoring of Tbo-filgrastim in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Tbo-filgrastim in the drug label.
Overdosage
No case of overdose has been reported.
Pharmacology
There is limited information regarding Tbo-filgrastim Pharmacology in the drug label.
Mechanism of Action
- Tbo-filgrastim is a human granulocyte colony-stimulating factor (G-CSF) produced by recombinant DNA technology. Tbo-filgrastim binds to G-CSF receptors and stimulates proliferation of neutrophils. G-CSF is known to stimulate differentiation commitment and some end-cell functional activation, which increases neutrophil counts and activity.
Structure
- Tbo-filgrastim is a non-glycosylated recombinant methionyl human granulocyte colony-stimulating growth factor (r-metHuG-CSF) manufactured by recombinant DNA technology using the bacterium strain E coli K802. It has a molecular weight of approximately 18.8 kDa and is composed of 175 amino acids. The endogenous human G-CSF is glycosylated and does not have the additional methionine amino acid residue in its NH2 terminal end.
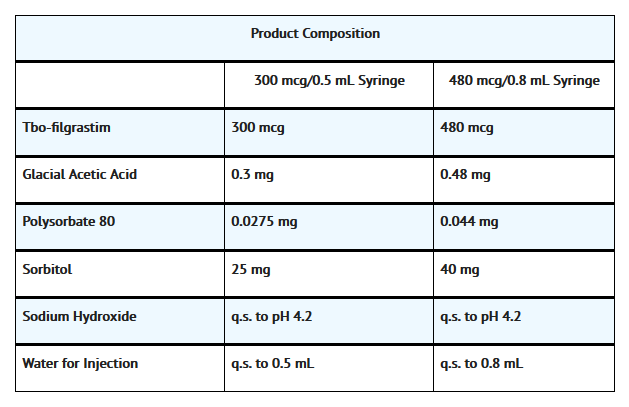
- The product is a sterile, clear, colorless, preservative-free solution containing tbo-filgrastim, glacial acetic acid, sorbitol, polysorbate 80, sodium hydroxide, and Water for Injection. The product is available in single-use prefilled syringes that contain either 300 mcg or 480 mcg of tbo-filgrastim at a fill volume of 0.5 mL or 0.8 mL, respectively. See table below for product composition of each single-use prefilled syringe.
Pharmacodynamics
- In the clinical trials of patients with cancer, the time to the ANCmax was between 3 to 5 days and returned to baseline by 21 days following completion of chemotherapy. In the healthy volunteer trials, doubling the tbo-filgrastim subcutaneous dose from 5 to 10 mcg/kg resulted in a 16%-19% increase in the ANCmax and a 33%-36% increase in the area under the effect curve for ANC.
Cardiac Electrophysiology
- At the maximum recommended intravenous dose of 5 μg/kg, tbo-filgrastim did not prolong the QT interval to any clinically relevant extent.
Pharmacokinetics
- In healthy subjects, the absolute bioavailability of 5 mcg/kg subcutaneous tbo-filgrastim was 33%. Increasing the dose of tbo-filgrastim from 5 to 10 mcg/kg in these healthy subjects resulted in an approximately 200% increase in both the maximum concentration (Cmax) and the area under the curve (AUC0-48h) of the drug.
- In the clinical trials of patients with cancer, the AUC and Cmax were greater and more variable compared to healthy volunteers receiving the same dose of tbo-filgrastim subcutaneously. The median time to maximum concentration was between 4 to 6 hours and the median elimination half-life was between 3.2 to 3.8 hours. Accumulation was not observed after repeated dosing.
Pharmacokinetics in Specific Populations
- Age: Not evaluated.
- Gender: No gender-related differences were observed.
- Renal Impairment: Mild renal impairment (creatinine clearance 60 - 89 mL/min) had no effect on tbo-filgrastim pharmacokinetics (N=11). The pharmacokinetic profile in patients with moderate and severe renal impairment has not been assessed.
- Hepatic Impairment: The pharmacokinetic profile in patients with hepatic impairment has not been assessed.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Carcinogenicity and genetic toxicology studies have not been conducted with tbo-filgrastim.
- A fertility study was not conducted with tbo-filgrastim. Toxicology studies of up to 26 weeks in rats or monkeys did not reveal findings in male or female reproductive organs that would suggest impairment of fertility.
Clinical Studies
- The efficacy of Tbo-filgrastim was evaluated in a multinational, multicenter, randomized and controlled Phase 3 study in 348 chemotherapy-naive patients with high-risk stage II, stage III, or stage IV breast cancer receiving doxorubicin (60 mg/m2) and docetaxel (75 mg/m2) comparing Tbo-filgrastim to placebo and a non-US-approved filgrastim product as controls. The median age of the patients was 50 years (range 25 to 75 years) with 99% female and 86% Caucasian.
- Tbo-filgrastim, placebo, and the non-US-approved filgrastim product were administered at 5 mcg/kg subcutaneously once daily beginning one day after chemotherapy for at least five days and continued to a maximum of 14 days or until an ANC of ≥10,000 x 106/L after nadir was reached.
- Tbo-filgrastim was superior to placebo in duration of severe neutropenia (DSN) with a statistically significant reduction in DSN (1.1 days vs. 3.8 days, p < 0.0001).
How Supplied
- Tbo-filgrastim solution for injection is supplied as a single-use, preservative-free, prefilled syringe of Type I glass which has a permanently attached stainless steel needle. Syringes may be supplied with or without an UltraSafe Passive® Needle Guard.
- The active substance is tbo-filgrastim.
- Tbo-filgrastim 300 mcg/0.5 mL: Each prefilled syringe contains 300 mcg of tbo-filgrastim in 0.5 mL solution with a blue plunger in:
- Pack of 1 with a safety needle guard in blister: NDC 63459-910-11
- Packs of 10 with a safety needle guard in blisters: NDC 63459-910-15
- Pack of 1 without a safety needle guard (for patients and caregivers): NDC 63459-910-17
- Packs of 5 without a safety needle guard (for patients and caregivers): NDC 63459-910-36
- Tbo-filgrastim 480 mcg/0.8 mL: Each prefilled syringe contains 480 mcg of tbo-filgrastim in 0.8 mL solution with a clear plunger in:
- Pack of 1 with a safety needle guard in blister: NDC 63459-912-11
- Packs of 10 with a safety needle guard in blisters: NDC 63459-912-15
- Pack of 1 without a safety needle guard (for patients and caregivers): NDC 63459-912-17
- Packs of 5 without a safety needle guard (for patients and caregivers): NDC 63459-912-36
Storage
- Tbo-filgrastim syringes should be stored in a refrigerator at 36° to 46° F (2° to 8° C). Protect from light. Within its shelf life, the product may be removed from 36° to 46° F (2° to 8° C) storage for a single period of up to 5 days between 73° to 81° F (23° to 27° C). If not used within 5 days, the product may be returned to 36° to 46° F (2° to 8° C) up to the expiration date.
- Avoid shaking. The solution should be visually inspected prior to use. Only clear solutions without particles should be used. Exposure to 23° to 30° F (-1° to -5 °C) for up to 72 hours and temperatures as low as 5° to -13° F (-15 to -25° C) for up to 24 hours do not adversely affect the stability of Tbo-filgrastim.
- Single-use syringe – discard unused portion. Any unused product or waste material should be disposed of in accordance with local requirements.
Images
Drug Images
{{#ask: Page Name::Tbo-filgrastim |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Tbo-filgrastim |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Availability of Patient Information and Instructions for Use
- Advise all patients and/or caregivers to read the FDA-approved Patient Information. For patients that are candidates for self-administration, assist patients and caregivers in understanding the contents of the Patient Information as well as the Tbo-filgrastim Instructions for Use that are included with the product, and give them the opportunity to ask questions prior to initiating therapy.
Patient Training
- Once it is determined that a patient is an appropriate candidate for self-administration or administration by a caregiver, instruct the patient or caregivers on the proper storage, preparation, and administration technique for Tbo-filgrastim. Advise the patients to read the FDA-approved Patient Information and Instructions for Use for further information.
Bone Pain
- Bone pain is common. Analgesics such as acetaminophen or NSAIDS may be necessary.
Rupture or Enlargement of Spleen
- Rupture or enlargement of the spleen may occur, which may be signaled by abdominal pain, left upper quadrant pain, or left shoulder pain. Advise patients to report onset of pain in these areas to their doctor immediately.
Dyspnea
- Dyspnea with or without fever, progressing to Acute Respiratory Distress Syndrome, may occur. Advise patients to report dyspnea immediately to their doctor.
Allergic Reactions
- Serious allergic reactions, including anaphylaxis, rash, and urticaria: Patients should report such reactions immediately to their doctor.
- In patients with sickle cell disorders, sickle cell crisis and death has occurred. Discuss the potential risks and benefits for patients with sickle cell disorders prior to the administration of human Granulocyte colony-stimulating factor.
Infections
- Tbo-filgrastim is used in circumstances where the risk of infection is increased. Patients should be alert for signs of infection such as fever, redness or swelling, and should report these findings to their doctor immediately.
Pregnancy
- Inform patients not to become pregnant while receiving Tbo-filgrastim. If pregnancy occurs, advise patients of the possibility of fetal harm.
Precautions with Alcohol
- Alcohol-Tbo-filgrastim interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- GRANIX®
Look-Alike Drug Names
There is limited information regarding Tbo-filgrastim Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Tbo-filgrastim
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Tbo-filgrastim |Label Name=Tbo-filgrastim04.png
}}
{{#subobject:
|Label Page=Tbo-filgrastim |Label Name=Tbo-filgrastim05.png
}}
{{#subobject:
|Label Page=Tbo-filgrastim |Label Name=Tbo-filgrastim06.png
}}
{{#subobject:
|Label Page=Tbo-filgrastim |Label Name=Tbo-filgrastim07.png
}}
{{#subobject:
|Label Page=Tbo-filgrastim |Label Name=Tbo-filgrastim08.png
}}
{{#subobject:
|Label Page=Tbo-filgrastim |Label Name=Tbo-filgrastim09.png
}}
{{#subobject:
|Label Page=Tbo-filgrastim |Label Name=Tbo-filgrastim10.png
}}