Tay-Sachs disease
|
WikiDoc Resources for Tay-Sachs disease |
|
Articles |
|---|
|
Most recent articles on Tay-Sachs disease Most cited articles on Tay-Sachs disease |
|
Media |
|
Powerpoint slides on Tay-Sachs disease |
|
Evidence Based Medicine |
|
Cochrane Collaboration on Tay-Sachs disease |
|
Clinical Trials |
|
Ongoing Trials on Tay-Sachs disease at Clinical Trials.gov Trial results on Tay-Sachs disease Clinical Trials on Tay-Sachs disease at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Tay-Sachs disease NICE Guidance on Tay-Sachs disease
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Tay-Sachs disease Discussion groups on Tay-Sachs disease Patient Handouts on Tay-Sachs disease Directions to Hospitals Treating Tay-Sachs disease Risk calculators and risk factors for Tay-Sachs disease
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Tay-Sachs disease |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief:
For patient information click here
| Tay-Sachs disease | |
 | |
|---|---|
| Tay-Sachs Disease: Retina; Cherry Red Spot | |
| ICD-10 | E75.0 |
| ICD-9 | 330.1 |
| OMIM | 272800 272750 |
| DiseasesDB | 12916 |
| MedlinePlus | 001417 |
Overview
Tay-Sachs disease (abbreviated TSD, also known as GM2 gangliosidosis, Hexosaminidase A deficiency or Sphingolipidosis) is a genetic disorder, fatal in its most common variant known as Infantile Tay-Sachs disease. TSD is inherited in an autosomal recessive pattern. The disease occurs when harmful quantities of a fatty acid derivative called a ganglioside accumulate in the nerve cells of the brain. Gangliosides are lipids, components of cellular membranes, and the ganglioside GM2, implicated in Tay-Sachs disease, is especially common in the nervous tissue of the brain.
The disease is named after the British ophthalmologist Warren Tay who first described the red spot on the retina of the eye in 1881,[1] and the American neurologist Bernard Sachs[2] who described the cellular changes of Tay-Sachs and noted an increased prevalence in the Eastern European Jewish (Ashkenazi) population in 1887.
Research in the late 20th century demonstrated that Tay-Sachs disease is caused by mutations on the HEXA gene on chromosome 15. A large number of HEXA mutations have been discovered, and new ones are still being reported. These mutations reach significant frequencies in several populations. French Canadians of southeastern Quebec have a carrier frequency similar to Ashkenazi Jews, but they carry a different mutation. Many Cajuns of southern Louisiana carry the same mutation that is most common in Ashkenazi Jews. Most HEXA mutations are rare, and do not occur in genetically isolated populations. The disease can potentially occur from the inheritance of two unrelated mutations in the HEXA gene, one from each parent.[3]
Tay-Sachs disease is a rare disease. Other autosomal disorders such as cystic fibrosis and sickle cell anemia are far more common. The importance of Tay-Sachs lies in the fact that an inexpensive enzyme assay test was discovered and subsequently automated, providing one of the first "mass screening" tools in medical genetics.[4][5] In this way, it became a research and public health model for understanding and preventing all autosomal genetic disorders.
Historical Perspective
Tay-Sachs disease has become a model for the prevention of all genetic diseases. In the United States before 1970, the disease affected about 50–70 infants each year in Ashkenazi Jewish families. About 10 cases occurred each year in infants from families without identifiable risk factors. Before 1970, the disease had never been diagnosed at the time of birth. Physicians saw the disease for the first time in infants that failed to thrive, and they could do nothing for the parents or family. Although the genetic basis of the disease was understood, antenatal testing was not available, and families with a Tay-Sachs infant faced a one and four probability of another devastating outcome with each future pregnancy.[5]
Michael Kaback, a medical resident in pediatric neurology at Johns Hopkins University, saw two Tay-Sachs families in 1969. At the time, researchers had just uncovered the biochemical basis of the disease as the failure of an enzyme in a critical metabolic pathway. Kaback developed and later automated an enzyme assay test for detecting heterozygotes (carriers). This inexpensive test proved statistically reliable, with low rates of both errors and false positives. For the first time in medical history, it was possible to screen broadly for a genetic disease, and a physician or medical professional could counsel a family on strategies for prevention. Within a few decades, the disease had been virtually eliminated among Ashkenazi Jews. Most cases today are in families that do not have identifiable risk factors.[5]
Kaback and his associates also developed the first mass screening program for genetic disease. Every aspect of this landmark study was meticulously planned, including community liaison, blood-draw procedure, laboratory set-up, assay protocol, and follow-up genetic counseling. On a Sunday in May 1971, more than 1800 young adults of Ashkenazi Jewish ancestry in the Baltimore and Washington D.C. area were voluntarily screened for carrier status.[6] The success of the program demonstrated the efficacy of voluntary screening of an identifiable at-risk populations. Within a few years, these screening programs had been repeated among Ashkenazi Jews throughout the United States, Canada, western Europe, and Israel.[7][8][9]
In the first 30 years of testing, from 1969 through 1998, more than 1.3 million persons were tested, and 48,864 carriers were identified. In at-risk families, among couples where both husband and wife were carriers, more than 3000 pregnancies were monitored by amniocentesis or chorionic villus sampling. Out of 604 monitored pregnancies where where there was a prenatal diagnosis of Tay-Sachs disease, 583 pregnancies were terminated. Of the 21 pregnancies that were not terminated, 20 of the infants went on to develop classic infantile Tay-Sachs disease, and the 21st case progressed later to adult-onset Tay-Sachs disease. In more than 2500 pregnancies, at-risk families were assured that their children would not be affected by Tay-Sachs disease. Only three fetuses with infantile TSD were incorrectly diagnosed as being unaffected.[5]
Classification
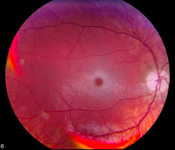
Tay-Sachs disease is classified in variant forms, based on the time of onset of neurological symptoms.[10] The variant forms reflect diversity in the mutation base.
All patients with Tay-Sachs disease have a "cherry-red" spot, easily observable by a physician using an ophthalmoscope, in the back of their eyes (the retina).[10] This red spot is the area of the retina which is accentuated because of gangliosides in the surrounding retinal ganglion cells (which are neurons of the central nervous system). The choroidal circulation is showing through "red" in this region of the fovea where all of the retinal ganglion cells are normally pushed aside to increase visual acuity. Thus, the cherry-red spot is the only normal part of the retina seen. Microscopic analysis of neurons shows that they are distended from excess storage of gangliosides.
- Infantile TSD. Infants with Tay-Sachs disease appear to develop normally for the first six months of life. Then, as nerve cells become distended with gangliosides, a relentless deterioration of mental and physical abilities occurs. The child becomes blind, deaf, and unable to swallow. Muscles begin to atrophy and paralysis sets in. Death usually occurs before the age of 4 or 5.[10]
- Juvenile TSD. Extremely rare, Juvenile Tay-Sachs disease usually presents itself in children between 2 and 10 years of age. They develop cognitive, motor, speech difficulties (dysarthria), swallowing difficulties (dysphagia), unsteadiness of gait (ataxia), and spasticity. Patients with Juvenile TSD usually die between 5–15 years.[11]
- Adult/Late Onset TSD. A rare form of the disorder, known as Adult Onset Tay-Sachs disease or Late Onset Tay-Sachs disease (LOTS), occurs in patients in their 20s and early 30s. LOTS is frequently misdiagnosed, and is usually non-fatal. It is characterized by unsteadiness of gait and progressive neurological deterioration. Symptoms of LOTS, which present in adolescence or early adulthood, include speech and swallowing difficulties, unsteadiness of gait, spasticity, cognitive decline, and psychiatric illness, particularly schizophrenic-like psychosis. Patients with LOTS frequently become full-time wheelchair users in adulthood, but many live full adult lives if psychiatric and physical difficulties are accommodated. Psychiatric symptoms and seizures can be controlled with medications.[12][13]
The development of improved testing methods has allowed neurologists to diagnosis Tay-Sachs and other neurological diseases with greater precision. Until the 1970s and 80s, when the molecular genetics of the disease became known, the juvenile and adult forms of the disease were not recognized as variants of Tay-Sachs. Post-infantile Tay-Sachs was often mis-diagnosed as another neurological disorder, such as Friedreich ataxia.[14]
Pathophysiology
File:Autorecessive.svg The condition is caused by insufficient activity of an enzyme called hexosaminidase A that catalyzes the biodegradation of fatty acid derivatives known as gangliosides. Hexasaminidase A is a vital hydrolytic enzyme, found in the lysosomes, that breaks down lipids. When Hexasaminidase A is no longer functioning properly, the lipids accumulate in the brain and cause problems.[10] Gangliosides are made and biodegraded rapidly in early life as the brain develops. Patients and carriers of Tay-Sachs disease can be identified by a simple blood test that measures hexosaminidase A activity. TSD is a recessive genetic disorder, meaning that both parents must be carriers in order to give birth to an affected child. Then, there is a 25% chance with each pregnancy of having a child with TSD. Prenatal monitoring of pregnancies is available.[10]
Hydrolysis of GM2-ganglioside requires three proteins. Two of them are subunits of hexosaminidase A, and the third is a small glycolipid transport protein, the GM2 activator protein (GM2A), which acts as a substrate specific cofactor for the enzyme. Deficiency in any one of these proteins leads to storage of the ganglioside, primarily in the lysosomes of neuronal cells. Tay-Sachs disease (along with GM2-gangliosidosis and Sandhoff disease) occurs because a genetic mutation inherited from both parents inactivates or inhibits this process. Most Tay-Sachs mutations appear not to affect functional elements of the protein. Instead, they cause incorrect folding or assembly of the enzyme, so that intracellular transport is disabled.[15]
The disease results from mutations on chromosome 15 in the HEXA gene encoding the alpha-subunit of the lysosomal enzyme beta-N-acetylhexosaminidase A. More than 90 mutations have been identified to date in the HEXA gene, and new mutations are still being reported. These mutations have included base pair insertions and deletions, splice site mutations, point mutations, and other more complex patterns. Each of these mutations alters the protein product, and thus inhibits the function of the enzyme in some manner. In recent years, population studies and pedigree analysis have shown how such mutations arise and spread within small founder populations.[3]
For example, a four base pair insertion in exon 11 (1278insTATC) results in an altered reading frame for the HEXA gene. This mutation is the most prevalent mutation in the Ashkenazi Jewish population, and leads to the infantile form of Tay-Sachs disease.[3] The same mutation occurs in the Cajun population of southern Louisiana, an American ethnic group that has been isolated for several hundred years because of linguistic differences. Researchers have traced carriers from several Louisiana families to a single founder couple, not known to be Jewish, that lived in France in the 18th century.[16]
An unrelated mutation, a long sequence deletion, occurs with similar frequency in families with French Canadian ancestry, and has the same pathological effects. Like the Ashkenazi Jewish population, the French Canadian population grew rapidly from a small founder group, and remained isolated from surrounding populations because of geographic, cultural, and language barriers. In the early days of Tay-Sachs research, the mutations in these two populations were believed to be identical. Some researchers claimed that a prolific Jewish ancestor must have introduced the mutation into the French Canadian population. This theory became known as the "Jewish Fur Trader Hypothesis" among researchers in population genetics. However, subsequent research has demonstrated that the two mutations are unrelated, and pedigree analysis has traced the French Canadian mutation to a founding family that lived in southern Quebec in the late 17th century.[17][18]
In the 1960s and early 1970s, when the biochemical basis of Tay-Sachs disease was first becoming known, no mutations had been sequenced directly for any genetic diseases. Researchers of that era did not yet know how common polymorphism would prove to be. The "Jewish Fur Trader Hypothesis," with its implication that a single mutation must have spread from one population into another, reflected the knowledge of the time. Subsequent research has proven that a large number of HEXA mutations can cause some form of the disease. Because Tay-Sachs disease was one of the first genetic disorders for which widespread genetic screening was possible, it is one of the first genetic disorders in which the prevalence of compound heterozygosity was demonstrated.[19]
Compound heterozygosity ultimately explains some of the variability of the disease, including late-onset forms. The disease can potentially result from the inheritance of two unrelated mutations in the HEXA gene, one from each parent. Classic infantile TSD results when a child has inherited mutations from both parents that completely inactivate the biodegradation of gangliosides. Late onset forms of the disease occur because of the diverse mutation base. Patients may technically be heterozygotes, but with two different HEXA mutations that both inactivate, alter, or inhibit enzyme activity in some way. When a patient has at least one copy of the HEXA gene that still enables some hexosaminidase A activity, a later onset form of the disease occurs. When disease occurs because of two unrelated mutations, the patient is said to be a compound heterozygote.[20]
Causes
Differentiating Tay-Sachs disease from Other Diseases
Epidemiology and Demographics
Historically, Eastern European people of Jewish descent (Ashkenazi Jews]]) have a high incidence of Tay-Sachs and other lipid storage diseases. Documentation of Tay-Sachs in this Jewish population reaches back to 15th century Europe. In the United States, about 1 in 27 to 1 in 30 Ashkenazi Jews is a recessive carrier. French Canadians and the Cajun community of Louisiana have an occurrence similar to the Ashkenazi Jews. Irish Americans have a 1 in 50 chance of a person being a carrier. In the general population, the incidence of carriers (heterozygotes) is about 1 in 300.[3]
A continuing controversy is whether heterozygotes, individuals who are carriers of one copy of the gene but do not actually develop the disease, have some selective advantage. The classic case of heterozygote advantage in humans is sickle cell anemia, and some researchers have argued that there must be some evolutionary benefit to being a heterozygote for Tay-Sachs as well.[21]
Four different theories have been proposed to explain the high frequency of Tay-Sachs carriers in the Ashkenazi Jewish population:
- Heterozygote advantage with tuberculosis resistance. In the 1970s and 80s, several researchers investigated whether being a Tay-Sachs carrier might have served as a form of protection against tuberculosis in medieval Europe. Tuberculosis was prevalent in the European Jewish populations, in part because Jews were forced to live in ghettos. However, several statistical studies have demonstrated that grandparents of Tay-Sachs carriers (who are more likely to have been carriers themselves) died proportionally from the same causes as non-carriers.[22]
- Heterozygote advantage because of higher intelligence. Another theory (attributed to Gregory Cochran) proposes that Tay-Sachs, and the other lipid storage diseases that are prevalent in Ashkenazi Jews, reflect genes that enhance dendrite growth and promote higher intelligence when present in carrier form. In this way, Cochran proposes that being a heterozygote provided a selective advantage at a time when Ashkenazi Jews were restricted to intellectual occupations.[23][24] (See Ashkenazi intelligence.)
- Reproductive compensation. Parents who lose a child because of disease tend to "compensate" by having additional children to replace them, and this may increase the incidence of autosomal recessive disease.[25]
- Founder effect. This hypothesis states that the high incidence of the 1278insTATC mutation is the result of genetic drift, which amplified a high frequency that existed by chance in an early founder population.[26][27][28]
Because Tay-Sachs disease was one of the first autosomal recessive genetic disorders for which there was an enzyme assay test (prior to polymerase chain reaction testing methods), it was intensely studied as a model for all such diseases. The researchers of the 1970s often favored theories of heterozygote advantage, but failed to find much evidence for them in human populations. They were also unaware of the diversity of the Tay-Sachs mutation base. In the 1970s, complete genomes had not yet been sequenced, and researchers were unaware of the extent of polymorphism. The contribution to evolution of genetic drift (as opposed to natural selection) was not fully appreciated.
Since the 1970s, DNA sequencing techniques using PCR have been applied to many genetic disorders, and in other human populations. Several broad genetic studies of the Ashkenazi population (not related to genetic disease) have demonstrated that the Ashkenazi Jews are the descendants of a small founder population, which may have gone through additional population bottlenecks. These studies also correlate well with historical information about Ashkenazi Jews. Thus, a preponderance of the recent studies have supported the founder effects theory.[26][27][28]
Risk Factors
Screening
Screening for Tay-Sachs disease was one of the first great successes of the emerging field of genetic counseling and diagnosis. Jewish communities, both inside and outside of Israel, embraced the cause of genetic screening from the 1970s on. Success with Tay-Sachs disease lead Israel to become the first country to offer free genetic screening and counseling for all couples. Israel has become a leading center for research on genetic disease. Both the Jewish and Arab/Palestinian populations in Israel contain many ethnic and religious minority groups, and Israel's initial success with Tay-Sachs disease has led to the development of screening programs for other diseases. Israel's success with Tay-Sachs disease has also opened several discussions and debates about the proper scope of genetic testing for other disorders.[29]
Genetic screening for carriers of Tay-Sachs disease is possible because an inexpensive enzyme assay test is available. It detects lower levels of the enzyme hexosaminidase A in serum. Developed and then automated during the 1970s, the enzyme assay test is not as precise as genetic testing based on polymerase chain reaction (PCR) techniques; however, it is cost effective for much broader use and allows screening for a disease that is rare in most populations. Couples with positive or ambiguous test results on the enzyme assay test may be referred for more precise screening. Current testing methods screen a panel of the most common mutations, although this leaves open a small probability of both false positive and false negative results. PCR testing is more effective when the ancestry of both parents is known, allowing for proper selection of genetic markers. Genetic counselors, working with couples that plan to conceive a child, assess risk factors based on ancestry to determine which testing methods are appropriate.Closing </ref> missing for <ref> tag
Proactive testing has been quite effective in eliminating Tay-Sachs occurrence among Ashkenazi Jews, both in Israel and in the diaspora. On January 18, 2005, the Israeli English language daily Haaretz reported that as a "Jewish disease" Tay-Sachs had almost been eradicated. Of the 10 babies born with Tay-Sachs in North America in 2003, none had been born to Jewish families. In Israel, only one child was born with Tay-Sachs in 2003, and preliminary results from early 2005 indicated that none were born with the disease in 2004.[30]
Natural History, Complications, and Prognosis
Diagnosis
Diagnostic Criteria
History and Symptoms
Physical Examination
Laboratory Findings
Imaging Findings
Other Diagnostic Studies
Treatment
Medical Therapy
There is currently no cure or treatment for TSD. Even with the best care, children with Infantile TSD die by the age of 5, and the progress of Late-Onset TSD can only be slowed, not reversed. Since Tay-Sachs disease is a lysosomal storage disorder, the research strategies have been those for lysosomal storage disorders in general. Several methods of treatment have been investigated for Tay-Sachs disease, but none have passed the experimental stage:
- Enzyme replacement therapy. Several ERT techniques have been investigated for lysosomal storage disorders, and could potentially be used to treat Tay-Sachs disease.[31] The goal would be to replace the missing enzyme, a process similar to insulin injections for diabetes. However, the HEXA enzyme has proven to be too large to pass through the blood into the brain through the blood-brain barrier. Blood vessels in the brain develop junctions so small that many toxic (or large) molecules cannot enter into nerve cells and cause damage. Researchers have also tried instilling the enzyme into cerebrospinal fluid, which bathes the brain. However, neurons are unable to take up the large enzyme efficiently even when it is placed next to the cell, so the treatment is still ineffective.
- Gene therapy. Several options for gene therapy have been explored for Tay-Sachs and other lysosomal storage diseases. If the defective genes could be replaced throughout the brain, Tay-Sachs could theoretically be cured. However, researchers working in this field believe that they are years away from the technology to transport the genes into neurons, which would be as difficult as transporting the enzyme. Use of a viral vector, promoting an infection as a means to introduce new genetic material into cells, has been proposed as a technique for genetic diseases in general. Hematopoetic stem cell therapy (HSCT), another form of gene therapy, uses cells that have not yet differentiated and taken on specialized functions. Yet another approach to gene therapy uses stem cells from umbilical cord blood in an effort to replace the defective gene. Although the stem cell approach has been effective with Krabbé disease,[32] no results for this method have been reported with Tay-Sachs disease.
- Substrate reduction therapy. Other highly experimental methods being researched involve manipulating the brain's metabolism of GM2 gangliosides.[33] One experiment has demonstrated that, by using the enzyme sialidase, the genetic defect can be effectively bypassed and GM2 gangliosides can be metabolized so that they become almost inconsequential. If a safe pharmacological treatment can be developed, one that causes the increased expression of lysosomal sialidase in neurons, a new form of therapy, essentially curing the disease, could be on the horizon. Metabolic therapies under investigation for Late-Onset TSD include treatment with the drug OGT 918 (Zavesca).[34]
Surgery
Prevention
Three approaches have been used to prevent or reduce the incidence of Tay-Sachs disease in the Ashkenazi Jewish population:
- Prenatal diagnosis and selective abortion. If both parents are identified as carriers, prenatal genetic testing can determine whether the fetus has inherited a defective copy of the gene from both parents. For couples who are willing to terminate the pregnancy, this eliminates the risk of Tay-Sachs, but selective abortion raises ethical issues for many families.[35]
- Mate selection. In Orthodox Jewish circles, the organization Dor Yeshorim carries out an anonymous screening program so that couples who are likely to conceive a child with Tay-Sachs or another genetic disorder can avoid marriage.[36] Nomi Stone of Dartmouth College describes this approach. "Orthodox Jewish high school students are given blood tests to determine if they have the Tay-Sachs gene. Instead of receiving direct results as to their carrier status, each person is given a six-digit identification number. Couples can call a hotline, if both are carriers, they will be deemed 'incompatible.' Individuals are not told they are carriers directly to avoid any possibility of stigmatization or discrimination. If the information were released, carriers could potentially become unmarriageable within the community."[37] Anonymous testing eliminates the stigma of carriership while decreasing the rate of homozygosity in this population. Stone notes that this approach, while effective within a confined population such as Hasidic or Orthodox Jews, may not be effective in the general population.[37]
- Preimplantation genetic diagnosis. By retrieving the mother's eggs for in vitro fertilization and conceiving a child outside the womb, it is possible to test the embryo prior to implantation. Only healthy embryos are selected for transfer into the mother's womb. In addition to Tay-Sachs disease, PGD has been used to prevent cystic fibrosis, sickle cell anemia, Huntington disease, and other genetic disorders.[38] However this method is expensive. It requires invasive medical technologies, and is beyond the financial means of many couples.
External links
- National Tay-Sachs & Allied Diseases Association
- NINDS Tay-Sachs Disease Information Page
- tay-sachs.org
- Tay-Sachs disease at NLM Genetics Home Reference
References
- ↑ Enersen, Ole Daniel. Warren Tay. WhoNamedIt.com. Retrieved on 2007-05-10
- ↑ Enersen, Ole Daniel. Bernard (Barney) Sachs. WhoNamedIt.com. Retrieved on 2007-05-10
- ↑ 3.0 3.1 3.2 3.3 "Genedis: Human Genetics Disease Database". Tel Aviv University, Department of Human Genetics, Bioinformatics Unit. Retrieved 2007-05-11.
- ↑ O'Brine JS, Okada S, Chen A, Fillerup DL (1970). "Tay-Sachs disease. Detection of heterozygotes and homozygotes by serum hexaminidase assay." New England Journal of Medicine 283, pp 15-20.}}
- ↑ 5.0 5.1 5.2 5.3 Kaback MM (2001). "Screening and prevention in Tay-Sachs disease: origins, update, and impact". Advances in Genetics. 44: 253–65. PMID 11596988.
- ↑ Kaback, Michael M.; Zeiger, R.S. (1972), "Heterozygote detection in Tay-Sachs disease: A prototype community screening program for the prevention of recessive genetic disorders", Sphingolipids, Sphingolipidosis, and Allied Disorders: Advances in Experimental Medicine and Biology, Plenum, 10: 613–632
- ↑ Kaback, M.M., Zeiger, R.S., Reynolds, L.W., and Sonneborn, M. (1974) "Tay-Sachs disease: A model for the control of recessive genetic disorders," in Birth Defects, Proceedings of the Fourth International Conference. (A. Motulsky and W. Lenz, editors) Amsterdam: Excerpta Medica, pp. 248-262.
- ↑ Kaback, M.M., Zeiger, R.S., Reynolds, L.W., and Sonneborn, M. (1974) "Approaches to the control and prevention of Tay-Sachs disease," in Progress in Medical Genetics, 10 (A. G. Steinberg and A. Bearn, editors) New York: Grune & Stratton, pp 103-134.
- ↑ Kaback, M. M.; Rimoin, D. L.; O'Brien, J. S. (1977) Tay-Sachs Disease: Screening and Prevention. New York: Alan R. Liss.
- ↑ 10.0 10.1 10.2 10.3 10.4 "Tay-Sachs Disease Information Page". National Institute of Neurological Disorders and Stroke. February 14 2007. Retrieved 2007-05-10. Check date values in:
|date=(help) - ↑ Moe, MD, Paul G. & Tim A. Benke, MD, PhD (2005). "Neurologic & Muscular Disorders". Current Pediatric Diagnosis & Treatment (17th ed.).
- ↑ Rosebush PI, MacQueen GM, Clarke JT, Callahan JW, Strasberg PM, Mazurek MF. (1995). "Late-onset Tay-Sachs disease presenting as catatonic schizophrenia: diagnostic and treatment issues". Journal of Clinical Psychiatry. 56 (8): 347–53. PMID 7635850.
- ↑ Neudorfer O, Pastores GM, Zeng BJ, Gianutsos J, Zaroff CM, Kolodny EH (2005). "Late-onset Tay-Sachs disease: phenotypic characterization and genotypic correlations in 21 affected patients". Genetics in Medicine. 7 (2): 119–23. PMID 15714079.
- ↑ Willner JP, Grabowski GA, Gordon RE, Bender AN, and Desnick RJ (July 1981). "Chronic GM2 gangliosidosis masquerading as atypical Friedreich ataxia: clinical, morphologic, and biochemical studies of nine cases". Neurology (7): 787–98. PMID 6454083.
- ↑ Mahuran DJ (1999). "Biochemical consequences of mutations causing the GM2 gangliosidoses". Biochim Biophys Acta. 1455 (2–3): 105–38. PMID 10571007.
- ↑ McDowell GA, Mulest EH, Fabacher P, Shapira JE, Blitzer MG (1992). "The presence of two different infantile Tay-Sachs disease mutations in a Cajun population" (PDF). American Journal of Human Geneticcs. 51 (5): 1071–1077. PMID 1307230. Retrieved 2007-05-10.
- ↑ Keats BJ, Elston RC, Andermann E (1987). "Pedigree discriminant analysis of two French Canadian Tay-Sachs families". Genetic Epidemiology. 4 (2): 77–85. PMID 2953646.
- ↑ De Braekeleer M, Hechtman P, Andermann E, and Kaplan F (April 1992). "The French Canadian Tay-Sachs disease deletion mutation: identification of probable founders". Human Genetics. 89 (1): 83–87. PMID 1577470.
- ↑ Ohno, Kousaku and Suzuki, Kunihiko (1988-12-05). "Multiple Abnormal beta-Hexosaminidase alpha-Chain mRNAs in a Compound-Heterozygous Ashkenazi Jewish Patient with Tay-Sachs Disease" (PDF). Journal of Biological Chemistry. 263 (34). PMID 2973464. Retrieved 2007-05-11.
- ↑ Kaback, Michael M. "Hexosaminidase A Deficiency". GeneReviews. Retrieved 2007-05-11.
- ↑ Chakravarti A and Chakraborty R (May 1978). "Elevated frequency of Tay-Sachs disease among Ashkenazic Jews unlikely by genetic drift alone". American Journal of Human Genetics. 30 (3): 256–61. PMID 677122.
- ↑ Spyropoulos B, Moens PB, Davidson J, and Lowden JA (1981). "Heterozygote advantage in Tay-Sachs carriers?". American Journal of Human Genetics (3): 375–80. PMID 7246543. Unknown parameter
|vol=ignored (|volume=suggested) (help) - ↑ Gregory Cochran, Jason Hardy, and Henry Harpending. "Natural History of Ashkenazi Intelligence" (PDF). Unknown parameter
|accessyear=ignored (|access-date=suggested) (help); Unknown parameter|accessmonthday=ignored (help) - ↑ Wade, Nicholas (June 3 2005). "Researchers Say Intelligence and Diseases May Be Linked in Ashkenazic Genes". New York Times. Retrieved 2007-05-13. Check date values in:
|date=(help) - ↑ Koeslag, JH and Schach, SR (1984). "Tay-Sachs disease and the role of reproductive compensation in the maintenance of ethnic variations in the incidence of autosomal recessive disease". Annals of Human Genetics (3): 275–281. PMID 6465844. Unknown parameter
|vol=ignored (|volume=suggested) (help) - ↑ 26.0 26.1 Risch, N, Tang, H, Katzenstein, H, Ekstein, J (2003). "Geographic distribution of disease mutations in the Ashkenazi Jewish population supports genetic drift over selection". American Journal of Human Genetics. 72 (4): 812–822. PMID 12612865.
- ↑ 27.0 27.1 Frisch A, Colombo R, Michaelovsky E, Karpati M, Goldman B, Peleg L. (2004). "Origin and spread of the 1278insTATC mutation causing Tay-Sachs disease in Ashkenazi Jews: genetic drift as a robust and parsimonious hypothesis". Human Genetics. 114 (4): 366–76. PMID 14727180.
- ↑ 28.0 28.1 Slatkin, M (2004). "A population-genetic test of founder effects and implications for Ashkenazi Jewish diseases". American Journal of Human Genetics. 75 (2): 282–293. PMID 15208782.
- ↑ Sagi, M (1998). "Ethical aspects of genetic screening in Israel". Science in Context. 11: 419–429. PMID 15168671.
- ↑ Traubman, Tamara (2005-01-18). "Tay-Sachs, the 'Jewish Disease,' Almost Eradicated". Haaretz. Retrieved 2007-05-14. Check date values in:
|date=(help) - ↑ "United States Patent 6066626, Compositions and method for treating lysosomal storage disease". FreePatentsOnline.com. Retrieved 2007-05-10.
- ↑ Escolar ML, Poe MD, Provenzale JM, Richards KC; et al. (2005). "Transplantation of Umbilical-Cord Blood in Babies with Infantile Krabbe's Disease". New England Journal of Medicine (20): 2069–2081. PMID 15901860. Unknown parameter
|vol=ignored (|volume=suggested) (help) - ↑ Lachmann RH and Platt FM (2001). "Substrate reduction therapy for glycosphingolipid storage disorders". 10 (3). Expert Opinion Investigational Drugs: 455–66. PMID 11227045.
- ↑ Kolodny EH, Neudorfer O, Gianutsos J; et al. (2004). "Late-onset Tay-Sachs disease: natural history and treatment with OGT 918 (Zavesca[TM])". Journal of Neurochemistry. 90 (54). ISSN 0022-3042.
- ↑ Stoller, David (1997). "Prenatal Genetic Screening: The Enigma of Selective Abortion". Journal of Law and Health. 12. PMID 10182027.
- ↑ Ekstein, J and Katzenstein, H (2001). "The Dor Yeshorim story: community-based carrier screening for Tay-Sachs disease". Advances in Genetics. 44: 297–310. PMID 11596991.
- ↑ 37.0 37.1 Nomi Stone. "Erasing Tay-Sachs Disease". Unknown parameter
|accessyear=ignored (|access-date=suggested) (help); Unknown parameter|accessmonthday=ignored (help) - ↑ Marik, JJ (April 13 2005). "Preimplantation Genetic Diagnosis". eMedicine.com. Retrieved 2007-05-10. Check date values in:
|date=(help)
Template:Endocrine, nutritional and metabolic pathology
ca:Malaltia de Tay-Sachs de:Tay-Sachs-Syndrom it:Malattia di Tay-Sachs he:טאי זקס sk:Tayov-Sachsov syndróm fi:Tay-Sachsin tauti