Sumatriptan (injection)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Turky Alkathery, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Sumatriptan (injection) is a serotonin (5-HT1B/1D) receptor agonist that is FDA approved for the treatment of migraine with or without aura in adults. Common adverse reactions include injection site reactions, tingling, dizziness/vertigo, warm/hot sensation, burning sensation, feeling of heaviness, pressure sensation, flushing, feeling of tightness, and numbness.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Sumatriptan Injection is indicated in adults for (1) the acute treatment of migraine, with or without aura, and (2) the acute treatment of cluster headache.
- Limitations of Use:
- Use only if a clear diagnosis of migraine or cluster headache has been established.
- If a patient has no response to the first migraine attack treated with sumatriptan, reconsider the diagnosis of migraine before sumatriptan is administered to treat any subsequent attacks.
- Sumatriptan is not indicated for the prevention of migraine attacks.
Dosage
Dosing Information
- The maximum single recommended adult dose of Sumatriptan Injection for the acute treatment of migraine or cluster headache is 6 mg injected subcutaneously. For the treatment of migraine, if side effects are dose limiting, lower doses (1 to 5 mg) may be used. For the treatment of cluster headache, the efficacy of lower doses has not been established.
- The maximum cumulative dose that may be given in 24 hours is 12 mg, two 6-mg injections separated by at least 1 hour. A second 6-mg dose should only be considered if some response to a first injection was observed.
Administration Using the Sumatriptan STATdose Pen
- An autoinjector device (Sumatriptan STATdose Pen) is available for use with 4- and 6-mg prefilled syringe cartridges. With this device, the needle penetrates approximately 1/4 inch (5 to 6 mm). The injection is intended to be given subcutaneously, and intramuscular or intravascular delivery must be avoided. Instruct patients on the proper use of Sumatriptan STATdose Pen and direct them to use injection sites with an adequate skin and subcutaneous thickness to accommodate the length of the needle.
Administration of Doses of Sumatriptan Other Than 4 or 6 mg
- In patients receiving doses other than 4 or 6 mg, use the 6-mg single-dose vial; do not use the Sumatriptan STATdose Pen. Visually inspect the vial for particulate matter and discoloration before administration. Do not use if particulates and discolorations are noted.
Dosage Forms and Strengths
- Injection: 4- and 6-mg single-dose prefilled syringe cartridges for use with the Sumatriptan STATdose Pen
- Injection: 6-mg single-dose vial
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Sumatriptan (injection) in adult patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Sumatriptan (injection) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Safety and effectiveness of Sumatriptan Injection in pediatric patients under 18 years of age have not been established; therefore, Sumatriptan Injection is not recommended for use in patients under 18 years of age.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Sumatriptan (injection) in pediatric patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Sumatriptan (injection) in pediatric patients.
Contraindications
- Sumatriptan Injection is contraindicated in patients with:
- Ischemic coronary artery disease (CAD) (angina pectoris, history of myocardial infarction, or documented silent ischemia) or coronary artery vasospasm, including Prinzmetal’s angina.
- Wolff-Parkinson-White syndrome or arrhythmias associated with other cardiac accessory conduction pathway disorders.
- History of stroke or transient ischemic attack (TIA) because these patients are at a higher risk of stroke.
- History of hemiplegic or basilar migraine.
- Peripheral vascular disease.
- Ischemic bowel disease.
- Uncontrolled hypertension.
- Recent (i.e., within 24 hours) use of ergotamine-containing medication, ergot-type medication (such as dihydroergotamine or methysergide), or another 5-hydroxytryptamine 1 (5-HT 1) agonist.
- Concurrent administration of an MAO-A inhibitor or recent (within 2 weeks) use of an MAO-A inhibitor.
- Known hypersensitivity to sumatriptan.
- Severe hepatic impairment.
Warnings
Myocardial Ischemia, myocardial infarction, and Prinzmetal’s Angina
- The use of Sumatriptan Injection is contraindicated in patients with ischemic or vasospastic CAD. There have been rare reports of serious cardiac adverse reactions, including acute myocardial infarction, occurring within a few hours following administration of Sumatriptan Injection. Some of these reactions occurred in patients without known CAD. 5-HT1 agonists, including Sumatriptan Injection, may cause coronary artery vasospasm (Prinzmetal’s angina), even in patients without a history of CAD.
- Perform a cardiovascular evaluation in triptan-naive patients who have multiple cardiovascular risk factors (e.g., increased age, diabetes, hypertension, smoking, obesity, strong family history of CAD) prior to receiving Sumatriptan Injection. If there is evidence of CAD or coronary artery vasospasm, Sumatriptan Injection is contraindicated. For patients with multiple cardiovascular risk factors who have a negative cardiovascular evaluation, consider administering the first dose of Sumatriptan Injection in a medically supervised setting and performing an electrocardiogram (ECG) immediately following Sumatriptan Injection. For such patients, consider periodic cardiovascular evaluation in intermittent long-term users of Sumatriptan Injection.
- Evaluate patients with signs or symptoms suggestive of angina following Sumatriptan Injection for the presence of CAD or Prinzmetal’s angina before receiving additional doses of Sumatriptan Injection.
Arrhythmias
- Life-threatening disturbances of cardiac rhythm, including ventricular tachycardia and ventricular fibrillation leading to death, have been reported within a few hours following the administration of 5-HT1 agonists. Discontinue Sumatriptan Injection if these disturbances occur. Sumatriptan Injection is contraindicated in patients with Wolff-Parkinson-White syndrome or arrhythmias associated with other cardiac accessory conduction pathway disorders.
Chest, Throat, Neck, and/or Jaw Pain/Tightness/Pressure
- As with other 5-HT1 agonists, sensations of tightness, pain, pressure, and heaviness in the precordium, throat, neck, and jaw commonly occur after treatment with Sumatriptan Injection and are usually non-cardiac in origin. However, perform a cardiac evaluation if these patients are at high cardiac risk. The use of Sumatriptan Injection is contraindicated in patients shown to have CAD and those with Prinzmetal’s variant angina.
Cerebrovascular Events
- Cerebral hemorrhage, subarachnoid hemorrhage, and stroke have occurred in patients treated with 5-HT1 agonists, and some have resulted in fatalities. In a number of cases, it appears possible that the cerebrovascular events were primary, the 5-HT1 agonist having been administered in the incorrect belief that the symptoms experienced were a consequence of migraine when they were not. Also, patients with migraine may be at increased risk of certain cerebrovascular events (e.g., stroke, hemorrhage, TIA). Discontinue Sumatriptan Injection if a cerebrovascular event occurs.
- As with other acute migraine therapies, before treating headaches in patients not previously diagnosed as migraineurs, and in migraineurs who present with atypical symptoms, exclude other potentially serious neurological conditions. Sumatriptan Injection is contraindicated in patients with a history of stroke or TIA.
Other Vasospasm Reactions
- 5-HT1 agonists, including Sumatriptan Injection, may cause non-coronary vasospastic reactions, such as peripheral vascular ischemia, gastrointestinal vascular ischemia and infarction (presenting with abdominal pain and bloody diarrhea), splenic infarction, and Raynaud’s syndrome. Until further evaluation, Sumatriptan Injection is contraindicated in patients who experience symptoms or signs suggestive of non-coronary vasospasm reaction following the use of any 5-HT1 agonist.
- Reports of transient and permanent blindness and significant partial vision loss have been reported with the use of 5-HT1 agonists. Since visual disorders may be part of a migraine attack, a causal relationship between these events and the use of 5-HT1 agonists have not been clearly established.
Medication Overuse Headache
- Overuse of acute migraine drugs (e.g., ergotamine, triptans, opioids, combination of drugs for 10 or more days per month) may lead to exacerbation of headache (medication overuse headache). Medication overuse headache may present as migraine-like daily headaches, or as a marked increase in frequency of migraine attacks. Detoxification of patients, including withdrawal of the overused drugs, and treatment of withdrawal symptoms (which often includes a transient worsening of headache) may be necessary.
Serotonin Syndrome
- Serotonin syndrome may occur with triptans, including Sumatriptan Injection, particularly during coadministration with selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), and MAO inhibitors. Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination), and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). The onset of symptoms usually occurs within minutes to hours of receiving a new or a greater dose of a serotonergic medication. Discontinue Sumatriptan Injection if serotonin syndrome is suspected.
Increase in Blood Pressure
- Significant elevation in blood pressure, including hypertensive crisis with acute impairment of organ systems, has been reported on rare occasions in patients treated with 5-HT1 agonists, including patients without a history of hypertension. Monitor blood pressure in patients treated with sumatriptan. Sumatriptan Injection is contraindicated in patients with Uncontrolled hypertension.
Anaphylactic/Anaphylactoid Reactions
- Anaphylactic/anaphylactoid reactions have occurred in patients receiving sumatriptan. Such reactions can be life threatening or fatal. In general, anaphylactic reactions to drugs are more likely to occur in individuals with a history of sensitivity to multiple allergens. Sumatriptan Injection is contraindicated in patients with prior serious anaphylactic reaction.
Seizures
- Seizures have been reported following administration of sumatriptan. Some have occurred in patients with either a history of seizures or concurrent conditions predisposing to seizures. There are also reports in patients where no such predisposing factors are apparent. Sumatriptan Injection should be used with caution in patients with a history of epilepsy or conditions associated with a lowered seizure threshold.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- Migraine Headache: Table 1 lists adverse reactions that occurred in 2 US placebo-controlled clinical trials in migraine subjects [Studies 2 and 3] following either a single 6-mg dose of Sumatriptan Injection or placebo. Only reactions that occurred at a frequency of 2% or more in groups treated with Sumatriptan Injection 6 mg and that occurred at a frequency greater than the placebo group are included in Table 1.

a The sum of the percentages cited is greater than 100% because subjects may have experienced more than 1 type of adverse reaction. Only reactions that occurred at a frequency of 2% or more in groups treated with Sumatriptan Injection and occurred at a frequency greater than the placebo groups are included. b Includes injection site pain, stinging/burning, swelling, erythema, bruising, bleeding. The incidence of adverse reactions in controlled clinical trials was not affected by gender or age of the subjects. There were insufficient data to assess the impact of race on the incidence of adverse reactions.
- Cluster Headache: In the controlled clinical trials assessing the efficacy of Sumatriptan Injection as a treatment for cluster headache [Studies 4 and 5] no new significant adverse reactions were detected that had not already been identified in trials of sumatriptan in subjects with migraine.
- Overall, the frequency of adverse reactions reported in the trials of cluster headache was generally lower than in the migraine trials. Exceptions include reports of paresthesia (5% Sumatriptan, 0% placebo), nausea and vomiting (4% Sumatriptan, 0% placebo), and bronchospasm (1% Sumatriptan, 0% placebo).
- Other Adverse Reactions: In the paragraphs that follow, the frequencies of less commonly reported adverse reactions are presented. Reaction frequencies were calculated as the number of subjects reporting a reaction divided by the total number of subjects (N = 6,218) exposed to subcutaneous Sumatriptan Injection. All reported reactions are included except those already listed in the previous table. Reactions are further classified within body system categories and enumerated in order of decreasing frequency using the following definitions: frequent are defined as those occurring in at least 1/100 subjects, infrequent are those occurring in 1/100 to 1/1,000 subjects, and rare are those occurring in fewer than 1/1,000 subjects.
- Cardiovascular: Infrequent were hypertension, hypotension, bradycardia, tachycardia, palpitations, and syncope. Rare was arrhythmia.
- Gastrointestinal: Frequent was abdominal discomfort.
- Musculoskeletal: Frequent were muscle cramps.
Rare were myoclonia, sleep disturbance, and dystonia.
- Respiratory: Infrequent was dyspnea.
- Miscellaneous: Infrequent was “serotonin agonist effect”.
- Adverse Events Observed With Other Formulations of Sumatriptan: The following adverse events occurred in clinical trials with Sumatriptan Tablets and Sumatriptan Nasal Spray. Because the reports include events observed in open and uncontrolled trials, the role of sumatriptan in their causation cannot be reliably determined. All reported events are included except those already listed, those too general to be informative, and those not reasonably associated with the use of the drug.
- Cardiovascular: Angina, cerebrovascular lesion, heart block, peripheral cyanosis, phlebitis, thrombosis.
- Gastrointestinal: Abdominal distention and colitis.
- Neurological: Convulsions, hallucinations, syncope, suicide, and twitching.
- Miscellaneous: Edema, hypersensitivity, swelling of extremities, and swelling of face.
Postmarketing Experience
- The following adverse reactions have been identified during postapproval use of Sumatriptan Tablets, Sumatriptan Nasal Spray, and Sumatriptan Injection. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These reactions have been chosen for inclusion due to either their seriousness, frequency of reporting, or causal connection to sumatriptan or a combination of these factors.
- Blood: Hemolytic anemia, pancytopenia, thrombocytopenia.
- Ear, Nose, and Throat: Deafness.
- Eye: Ischemic optic neuropathy, retinal artery occlusion, retinal vein thrombosis.
- Neurological: Central nervous system vasculitis, cerebrovascular accident, serotonin syndrome, subarachnoid hemorrhage.
- Non-Site Specific: Angioedema, cyanosis, temporal arteritis.
- Skin: Exacerbation of sunburn, hypersensitivity reactions (allergic vasculitis, erythema, pruritus, rash, shortness of breath, urticaria), photosensitivity. Following subcutaneous administration of sumatriptan, pain, redness, stinging, induration, swelling, contusion, subcutaneous bleeding, and, on rare occasions, lipoatrophy (depression in the skin) or lipohypertrophy (enlargement or thickening of tissue) have been reported.
- Urogenital: Acute renal failure.
Drug Interactions
Ergot-Containing Drugs
- Ergot-containing drugs have been reported to cause prolonged vasospastic reactions. Because these effects may be additive, use of ergotamine-containing or ergot-type medications (like dihydroergotamine or methysergide) and Sumatriptan Injection within 24 hours of each other is contraindicated.
Monoamine Oxidase-A Inhibitors
- MAO-A inhibitors increase systemic exposure by 2-fold. Therefore, the use of Sumatriptan Injection in patients receiving MAO-A inhibitors is contraindicated.
Other 5-HT 1 Agonists
- Because their vasospastic effects may be additive, coadministration of Sumatriptan Injection and other 5-HT1 agonists (e.g., triptans) within 24 hours of each other is contraindicated.
Selective Serotonin Reuptake Inhibitors/Serotonin Norepinephrine Reuptake Inhibitors and Serotonin Syndrome
- Cases of serotonin syndrome have been reported during coadministration of triptans and SSRIs, or SNRIs, TCAs, and MAO inhibitors.
Use in Specific Populations
Pregnancy
- There are no adequate and well-controlled trials of Sumatriptan Injection in pregnant women. Sumatriptan Injection should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- When sumatriptan was administered intravenously to pregnant rabbits daily throughout the period of organogenesis, embryolethality was observed at doses at or close to those producing maternal toxicity. These doses were less than the maximum recommended human dose (MRHD) of 12 mg/day on a mg/m2 basis. Oral administration of sumatriptan to rabbits during organogenesis was associated with increased incidences of fetal vascular and skeletal abnormalities. The highest no-effect dose for these effects was 15 mg/kg/day. The intravenous administration of sumatriptan to pregnant rats throughout organogenesis at doses that are approximately 10 times the MRHD on a mg/m2 basis, did not produce evidence of embryolethality. The subcutaneous administration of sumatriptan to pregnant rats prior to and throughout pregnancy did not produce evidence of embryolethality or teratogenicity.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Sumatriptan (injection) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Sumatriptan (injection) during labor and delivery.
Nursing Mothers
- It is not known whether sumatriptan is excreted in human breast milk following subcutaneous administration. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions in nursing infants from sumatriptan, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- Safety and effectiveness of Sumatriptan Injection in pediatric patients under 18 years of age have not been established; therefore, Sumatriptan Injection is not recommended for use in patients under 18 years of age.
- Two controlled clinical trials evaluated Sumatriptan Nasal Spray (5 to 20 mg) in 1,248 adolescent migraineurs aged 12 to 17 years who treated a single attack. The trials did not establish the efficacy of Sumatriptan Nasal Spray compared with placebo in the treatment of migraine in adolescents. Adverse reactions observed in these clinical trials were similar in nature to those reported in clinical trials in adults.
- Five controlled clinical trials (2 single-attack trials, 3 multiple-attack trials) evaluating oral Sumatriptan (25 to 100 mg) in pediatric subjects aged 12 to 17 years enrolled a total of 701 adolescent migraineurs. These trials did not establish the efficacy of oral Sumatriptan compared with placebo in the treatment of migraine in adolescents. Adverse reactions observed in these clinical trials were similar in nature to those reported in clinical trials in adults. The frequency of all adverse reactions in these subjects appeared to be both dose- and age-dependent, with younger subjects reporting reactions more commonly than older adolescents.
- Postmarketing experience documents that serious adverse reactions have occurred in the pediatric population after use of subcutaneous, oral, and/or intranasal Sumatriptan. These reports include reactions similar in nature to those reported rarely in adults, including stroke, visual loss, and death. A myocardial infarction has been reported in a 14-year-old male following the use of oral Sumatriptan; clinical signs occurred within 1 day of drug administration. Since clinical data to determine the frequency of serious adverse reactions in pediatric patients who might receive subcutaneous, oral, or intranasal Sumatriptan are not presently available, the use of Sumatriptan in patients under 18 years of age is not recommended.
Geriatic Use
- Clinical trials of Sumatriptan Injection did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger subjects. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy.
- A cardiovascular evaluation is recommended for geriatric patients who have other cardiovascular risk factors (e.g., diabetes, hypertension, smoking, obesity, strong family history of CAD) prior to receiving Sumatriptan Injection.
Gender
There is no FDA guidance on the use of Sumatriptan (injection) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Sumatriptan (injection) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Sumatriptan (injection) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Sumatriptan (injection) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Sumatriptan (injection) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Sumatriptan (injection) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Subcutaneous.
Monitoring
- Monitor blood pressure in patients treated with sumatriptan.
IV Compatibility
- There is limited information regarding IV Compatibility
Overdosage
- No gross overdoses in clinical practice have been reported. Coronary vasospasm was observed after intravenous administration of Sumatriptan Injection. Overdoses would be expected from animal data (dogs at 0.1 g/kg, rats at 2 g/kg) to possibly cause convulsions, tremor, inactivity, erythema of the extremities, reduced respiratory rate, cyanosis, ataxia, mydriasis, injection site reactions (desquamation, hair loss, and scab formation), and paralysis.
- The elimination half-life of sumatriptan is about 2 hours, and therefore monitoring of patients after overdose with Sumatriptan Injection should continue for at least 10 hours or while symptoms or signs persist.
- It is unknown what effect hemodialysis or peritoneal dialysis has on the serum concentrations of sumatriptan.
Pharmacology
Mechanism of Action
- Sumatriptan binds with high affinity to human cloned 5-HT1B/1D receptors. Sumatriptan presumably exerts its therapeutic effects in the treatment of migraine headache by binding to 5-HT1B/1D receptors located on intracranial blood vessels and sensory nerves of the trigeminal system.
- Current theories proposed to explain the etiology of migraine headache suggest that symptoms are due to local cranial vasodilatation and/or to the release of sensory neuropeptides (including substance P and calcitonin gene-related peptide) through nerve endings in the trigeminal system. The therapeutic activity of sumatriptan for the treatment of migraine and cluster headaches is thought to be due to the agonist effects at the 5-HT1B/1D receptors on intracranial blood vessels (including the arterio-venous anastomoses) and sensory nerves of the trigeminal system, which result in cranial vessel constriction and inhibition of pro-inflammatory neuropeptide release.
Structure
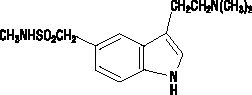
- Sumatriptan Injection contains sumatriptan succinate, a selective 5-HT1B/1D receptor agonist. Sumatriptan succinate is chemically designated as 3-[2-(dimethylamino)ethyl]-N-methyl-indole-5-methanesulfonamide succinate (1:1), and it has the following structure:
- The empirical formula is C14H21N3O2S•C4H6O4, representing a molecular weight of 413.5. Sumatriptan succinate is a white to off-white powder that is readily soluble in water and in saline.
- Sumatriptan Injection is a clear, colorless to pale yellow, sterile, nonpyrogenic solution for subcutaneous injection. Each 0.5 mL of Sumatriptan Injection 8 mg/mL solution contains 4 mg of sumatriptan (base) as the succinate salt and 3.8 mg of sodium chloride, USP in Water for Injection, USP. Each 0.5 mL of Sumatriptan Injection 12 mg/mL solution contains 6 mg of sumatriptan (base) as the succinate salt and 3.5 mg of sodium chloride, USP in Water for Injection, USP. The pH range of both solutions is approximately 4.2 to 5.3. The osmolality of both injections is 291 mOsmol.
Pharmacodynamics
- Blood Pressure: Significant elevation in blood pressure, including hypertensive crisis, has been reported in patients with and without a history of hypertension.
- Peripheral (Small) Arteries: In healthy volunteers (N = 18), a trial evaluating the effects of sumatriptan on peripheral (small vessel) arterial reactivity failed to detect a clinically significant increase in peripheral resistance.
- Heart Rate: Transient increases in blood pressure observed in some subjects in clinical trials carried out during sumatriptan’s development as a treatment for migraine were not accompanied by any clinically significant changes in heart rate.
Pharmacokinetics
- Absorption and Bioavailability: The bioavailability of sumatriptan via subcutaneous site injection to 18 healthy male subjects was 97% ± 16% of that obtained following intravenous injection.
- After a single 6-mg subcutaneous manual injection into the deltoid area of the arm in 18 healthy males (age: 24 ± 6 years, weight: 70 kg), the maximum serum concentration (Cmax) of sumatriptan was (mean ± standard deviation) 74 ± 15 ng/mL and the time to peak concentration (Tmax) was 12 minutes after injection (range: 5 to 20 minutes). In this trial, the same dose injected subcutaneously in the thigh gave a Cmax of 61 ± 15 ng/mL by manual injection versus 52 ± 15 ng/mL by autoinjector techniques. The Tmax or amount absorbed was not significantly altered by either the site or technique of injection.
- Distribution: Protein binding, determined by equilibrium dialysis over the concentration range of 10 to 1,000 ng/mL, is low, approximately 14% to 21%. The effect of sumatriptan on the protein binding of other drugs has not been evaluated.
- Following a 6-mg subcutaneous injection into the deltoid area of the arm in 9 males (mean age: 33 years, mean weight: 77 kg) the volume of distribution central compartment of sumatriptan was 50 ± 8 liters and the distribution half‑life was 15 ± 2 minutes.
- Metabolism: In vitro studies with human microsomes suggest that sumatriptan is metabolized by MAO, predominantly the A isoenzyme. Most of a radiolabeled dose of sumatriptan excreted in the urine is the major metabolite indole acetic acid (IAA) or the IAA glucuronide, both of which are inactive.
- Elimination: After a single 6-mg subcutaneous dose, 22% ± 4% was excreted in the urine as unchanged sumatriptan and 38% ± 7% as the IAA metabolite.
- Following a 6-mg subcutaneous injection into the deltoid area of the arm, the systemic clearance of sumatriptan was 1,194 ± 149 mL/min and the terminal half-life was 115 ± 19 minutes.
- Special Populations:Age: The pharmacokinetics of sumatriptan in the elderly (mean age: 72 years, 2 males and 4 females) and in subjects with migraine (mean age: 38 years, 25 males and 155 females) were similar to that in healthy male subjects (mean age: 30 years).
- Renal Impairment: The effect of renal impairment on the pharmacokinetics of sumatriptan has not been examined.
- Hepatic Impairment: The effect of mild to moderate hepatic disease on the pharmacokinetics of subcutaneously administered sumatriptan has been evaluated. There were no significant differences in the pharmacokinetics of subcutaneously administered sumatriptan in moderately hepatically impaired subjects compared with healthy controls. The pharmacokinetics of subcutaneously administered sumatriptan in patients with severe hepatic impairment has not been studied. The use of Sumatriptan Injection in this population is contraindicated.
- Race: The systemic clearance and Cmax of sumatriptan were similar in black (n = 34) and Caucasian (n = 38) healthy male subjects.
- Drug Interaction Studies: Monoamine Oxidase-A Inhibitors:In a trial of 14 healthy females, pretreatment with an MAO-A inhibitor decreased the clearance of sumatriptan, resulting in a 2-fold increase in the area under the sumatriptan plasma concentration-time curve (AUC), corresponding to a 40% increase in elimination half-life.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Carcinogenesis: In carcinogenicity studies, rats and mice were given sumatriptan by oral gavage. Mice were dosed for 78 weeks and rats were dosed for 104 weeks. Average exposures achieved in mice receiving the highest dose were approximately 110 times the exposure attained in humans after the maximum recommended single dose of 6 mg. The highest dose to rats was approximately 260 times the maximum single dose of 6 mg on a mg/m2 basis. There was no evidence of an increase in tumors in either species related to sumatriptan administration.
- Mutagenesis: Sumatriptan was not mutagenic in the presence or absence of metabolic activation when tested in 2 gene mutation assays (the Ames test and the in vitro mammalian Chinese hamster V79/HGPRT assay). It was not clastogenic in 2 cytogenetics assays (the in vitro human lymphocyte assay and the in vivo rat micronucleus assay).
- Impairment of Fertility: A fertility study (Segment I) by the subcutaneous route, during which male and female rats were dosed daily with sumatriptan prior to and throughout the mating period, has shown no evidence of impaired fertility at doses equivalent to approximately 100 times the maximum recommended single human dose of 6 mg on a mg/m2 basis. However, following oral administration, a treatment-related decrease in fertility, secondary to a decrease in mating, was seen for rats treated with 50 and 500 mg/kg/day. The no-effect dose for this finding was approximately 8 times the maximum recommended single human dose of 6 mg on a mg/m2 basis. It is not clear whether the problem is associated with the treatment of males or females or both.
Animal Toxicology and/or Pharmacology
- Corneal Opacities: Dogs receiving oral sumatriptan developed corneal opacities and defects in the corneal epithelium. Corneal opacities were seen at the lowest dosage tested, 2 mg/kg/day, and were present after 1 month of treatment. Defects in the corneal epithelium were noted in a 60-week study. Earlier examinations for these toxicities were not conducted and no-effect doses were not established; however, the relative exposure at the lowest dose tested was approximately 5 times the human exposure after a 100-mg oral dose or 3 times the human exposure after a 6-mg subcutaneous dose.
- Melanin Binding: In rats with a single subcutaneous dose (0.5 mg/kg) of radiolabeled sumatriptan, the elimination half-life of radioactivity from the eye was 15 days, suggesting that sumatriptan and its metabolites bind to the melanin of the eye. The clinical significance of this binding is unknown.
Clinical Studies
Migraine
- In controlled clinical trials enrolling more than 1,000 subjects during migraine attacks who were experiencing moderate or severe pain and 1 or more of the symptoms enumerated in Table 3, onset of relief began as early as 10 minutes following a 6-mg Sumatriptan Injection. Lower doses of Sumatriptan Injection may also prove effective, although the proportion of subjects obtaining adequate relief was decreased and the latency to that relief is greater with lower doses.
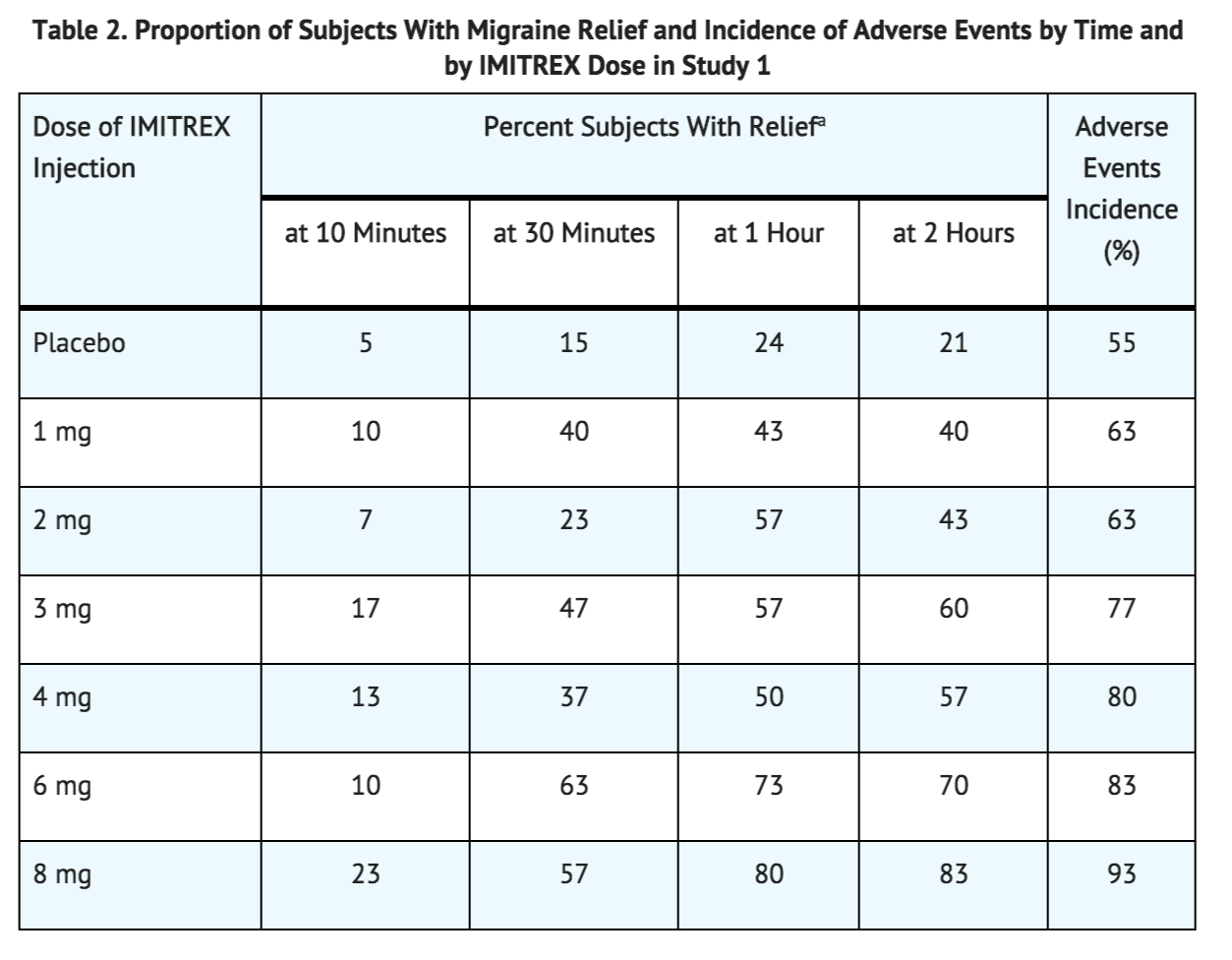
- In Study 1, 6 different doses of Sumatriptan Injection (n = 30 each group) were compared with placebo (n = 62), in a single-attack, parallel-group design, the dose response relationship was found to be as shown in Table 2.
a Relief is defined as the reduction of moderate or severe pain to no or mild pain after dosing without use of rescue medication.
- In 2 randomized, placebo-controlled clinical trials of Sumatriptan Injection 6 mg in 1,104 subjects with moderate or severe migraine pain (Studies 2 and 3), the onset of relief was less than 10 minutes. Headache relief, as defined by a reduction in pain from severe or moderately severe to mild or no headache, was achieved in 70% of the subjects within 1 hour of a single 6-mg subcutaneous dose of Sumatriptan Injection. Approximately 82% and 65% of subjects treated with Sumatriptan 6 mg had headache relief and were pain free within 2 hours, respectively.
- Table 3 shows the 1- and 2-hour efficacy results for Sumatriptan Injection 6 mg in Studies 2 and 3.
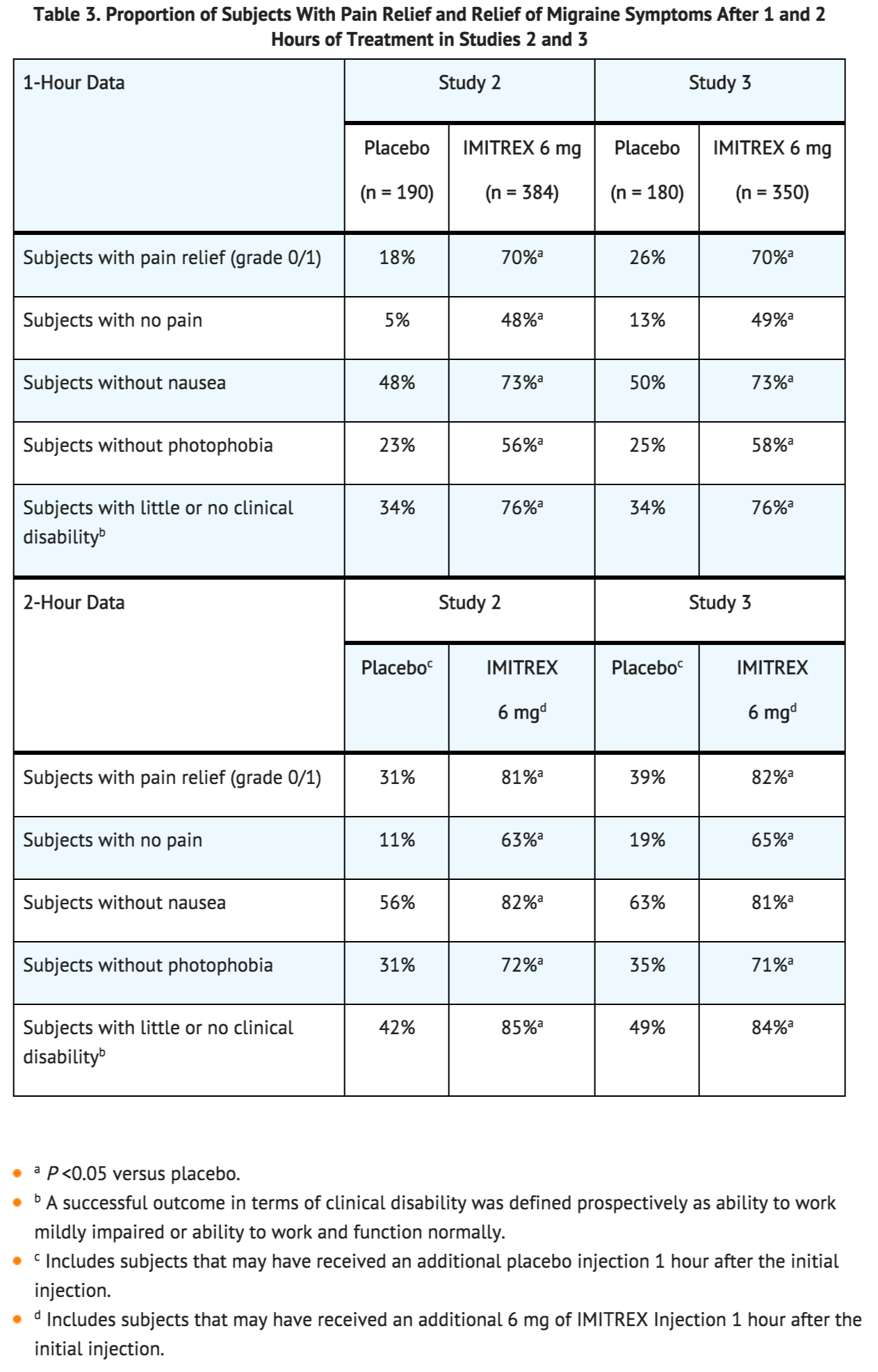
- Sumatriptan Injection also relieved photophobia, phonophobia (sound sensitivity), nausea, and vomiting associated with migraine attacks. Similar efficacy was seen when subjects self-administered Sumatriptan Injection using the Sumatriptan STATdose Pen.
- The efficacy of Sumatriptan Injection was unaffected by whether or not the migraine was associated with aura, duration of attack, gender or age of the subject, or concomitant use of common migraine prophylactic drugs (e.g., beta-blockers).
Cluster Headache
- The efficacy of Sumatriptan Injection in the acute treatment of cluster headache was demonstrated in 2 randomized, double-blind, placebo-controlled, 2-period crossover trials (Studies 4 and 5). Subjects aged 21 to 65 years were enrolled and were instructed to treat a moderate to very severe headache within 10 minutes of onset. Headache relief was defined as a reduction in headache severity to mild or no pain. In both trials, the proportion of individuals gaining relief at 10 or 15 minutes was significantly greater among subjects receiving 6 mg of Sumatriptan Injection compared with those who received placebo (see Table 4).
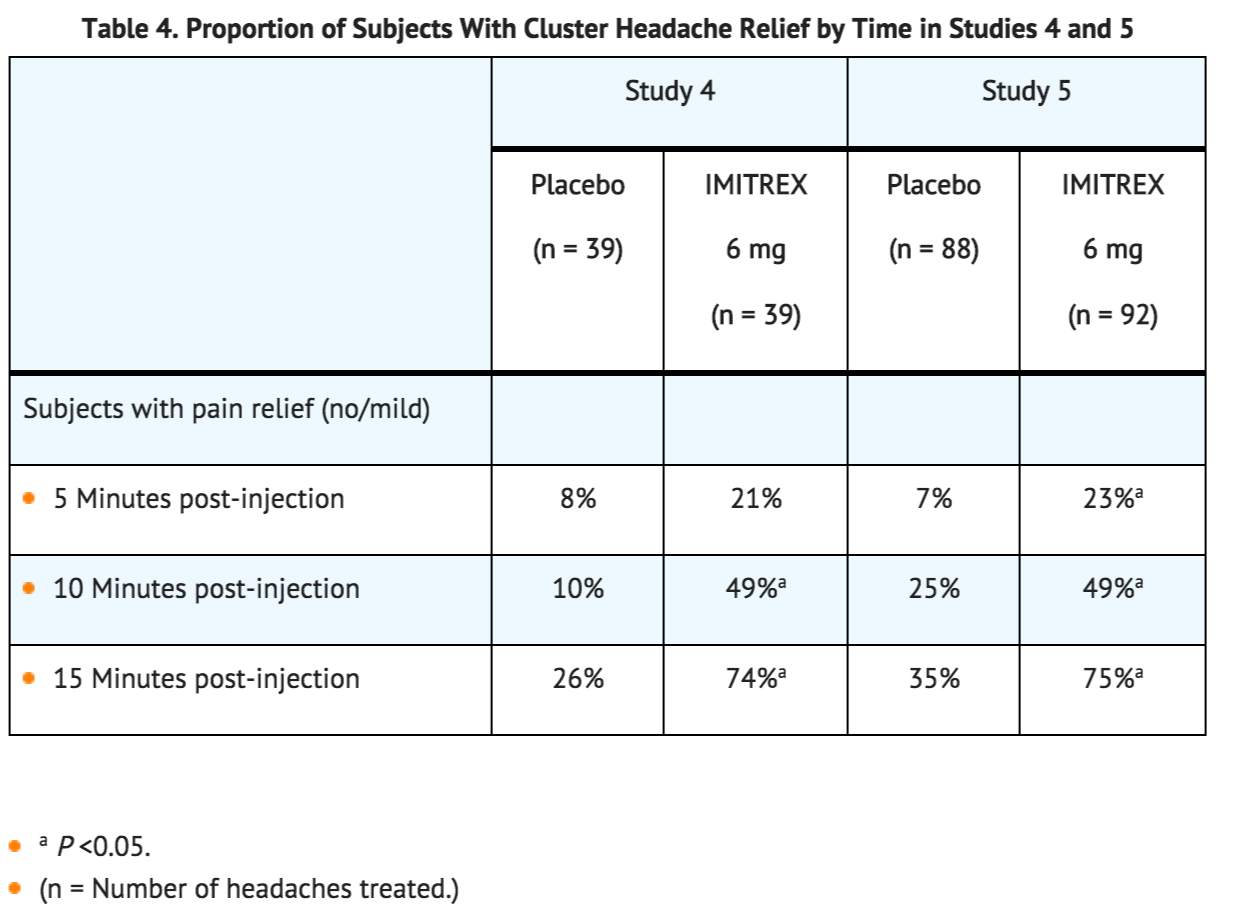
- An estimate of the cumulative probability of a subject with a cluster headache obtaining relief after being treated with either Sumatriptan Injection or placebo is presented in Figure 1.
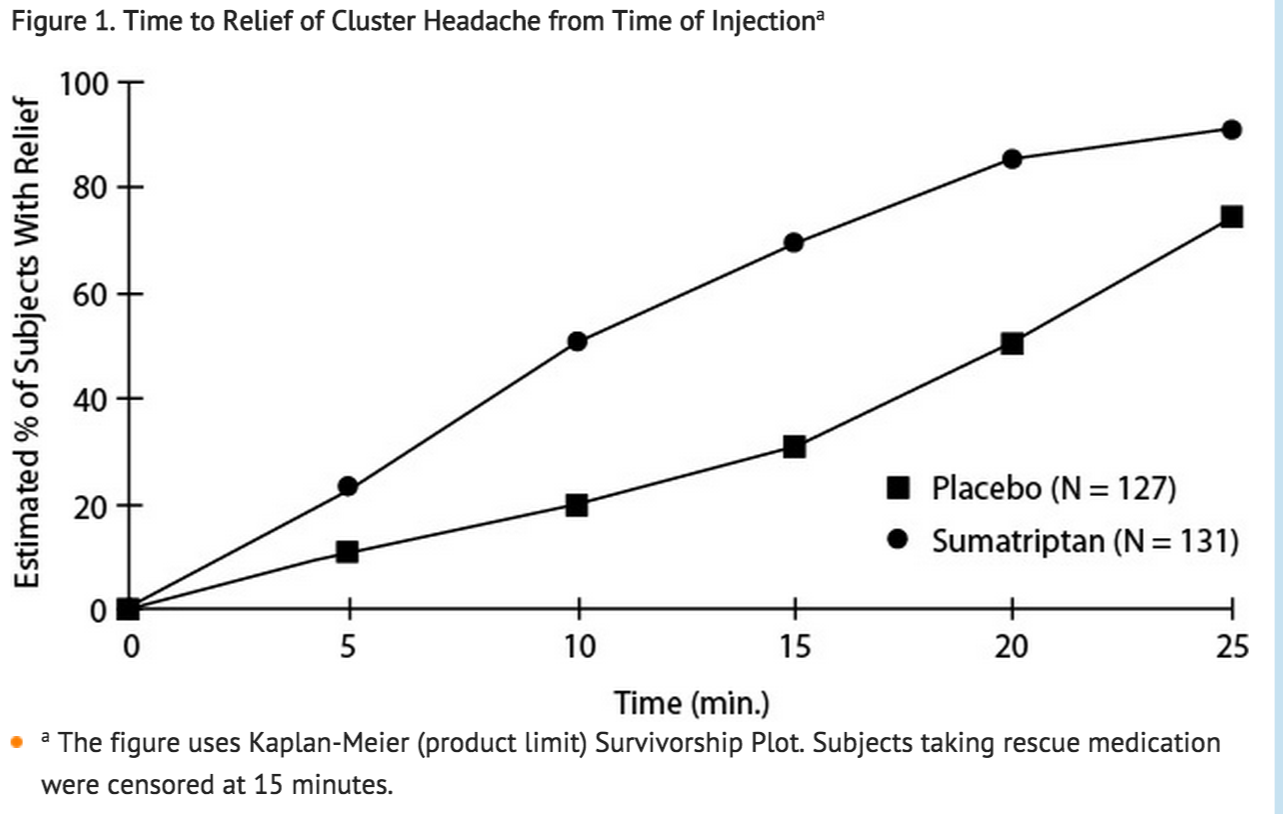
- The plot was constructed with data from subjects who either experienced relief or did not require (request) rescue medication within a period of 2 hours following treatment. As a consequence, the data in the plot are derived from only a subset of the 258 headaches treated (rescue medication was required in 52 of the 127 placebo-treated headaches and 18 of the 131 headaches treated with Sumatriptan Injection).
- Other data suggest that treatment with Sumatriptan Injection is not associated with an increase in early recurrence of headache and has little effect on the incidence of later-occurring headaches (i.e., those occurring after 2, but before 18 or 24 hours).
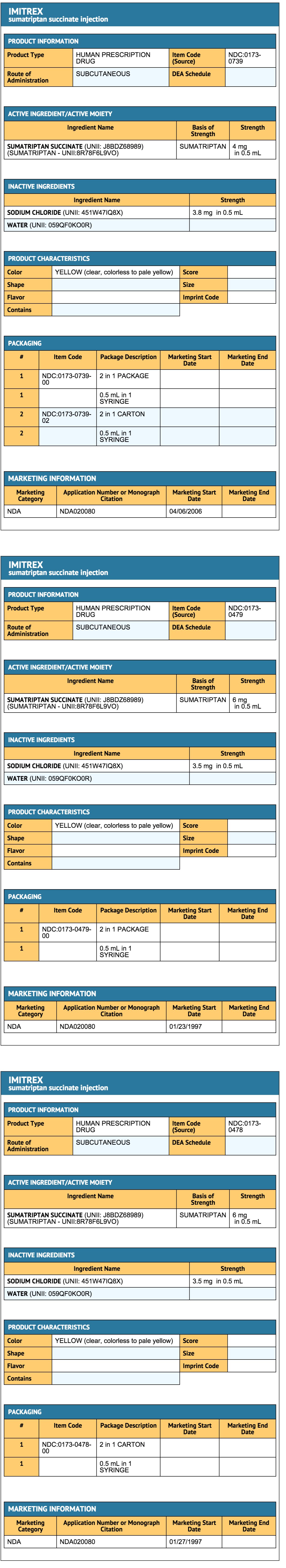
How Supplied
- MITREX Injection contains sumatriptan (base) as the succinate salt and is supplied as a clear, colorless to pale yellow, sterile, nonpyrogenic solution as follows:
- Prefilled Syringe and/or Autoinjector Pen: Each pack contains a Patient Information and Patients Instructions for Use leaflet.
- IMITREX STATdose System ®, 4 mg, containing 1 IMITREX STATdose Pen, 2 prefilled single-dose syringe cartridges, and 1 carrying case (NDC 0173-0739-00).
- IMITREX STATdose System, 6 mg, containing 1 IMITREX STATdose Pen, 2 prefilled single-dose syringe cartridges, and 1 carrying case (NDC 0173-0479-00).
- Two 4-mg single-dose prefilled syringe cartridges for use with IMITREX STATdose System (NDC 0173-0739-02).
- Two 6-mg single-dose prefilled syringe cartridges for use with IMITREX STATdose System (NDC 0173-0478-00).
- Single-Dose Vial:
- IMITREX Injection single-dose vial (6 mg/0.5 mL) in cartons containing 5 vials (NDC 0173-0449-02).
Storage
- Store between 2° and 30°C (36° and 86°F). Protect from light.
Images
Drug Images
{{#ask: Page Name::Sumatriptan (injection) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel



{{#ask: Label Page::Sumatriptan (injection) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Risk of Myocardial Ischemia and/or Infarction, Prinzmetal’s angina, Other Vasospasm-Related Events, Arrhythmias, and Cerebrovascular Events
- Inform patients that IMITREX Injection may cause serious cardiovascular side effects such as myocardial infarction or stroke. Although serious cardiovascular events can occur without warning symptoms, patients should be alert for the signs and symptoms of chest pain, shortness of breath, irregular heartbeat, significant rise in blood pressure, weakness, and slurring of speech and should ask for medical advice when observing any indicative sign or symptoms. Patients should be apprised of the importance of this follow-up [see Warnings and Precautions (5.1, 5.2, 5.4, 5.5, 5.8)].
Anaphylactic/Anaphylactoid Reactions
- Inform patients that anaphylactic/anaphylactoid reactions have occurred in patients receiving IMITREX Injection. Such reactions can be life threatening or fatal. In general, anaphylactic reactions to drugs are more likely to occur in individuals with a history of sensitivity to multiple allergens.
Medication Overuse Headache
- Inform patients that use of acute migraine drugs for 10 or more days per month may lead to an exacerbation of headache and encourage patients to record headache frequency and drug use (e.g., by keeping a headache diary).
Pregnancy
- Inform patients that IMITREX Injection should not be used during pregnancy unless the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
- Advise patients to notify their healthcare provider if they are breastfeeding or plan to breastfeed.
Ability To Perform Complex Tasks
- Since migraines or treatment with IMITREX Injection may cause somnolence and dizziness, instruct patients to evaluate their ability to perform complex tasks during migraine attacks and after administration of IMITREX Injection.
Serotonin Syndrome
- Patients should be cautioned about the risk of serotonin syndrome with the use of IMITREX Injection or other triptans, particularly during combined use with SSRIs, SNRIs, TCAs, and MAO inhibitors.
How to Use IMITREX Injection
- Provide patients instruction on the proper use of IMITREX Injection if they are able to self-administer IMITREX Injection in medically unsupervised situation.
- Inform patients that the needle in the IMITREX STATdose Pen penetrates approximately 1/4 of an inch (5 to 6 mm). Inform patients that the injection is intended to be given subcutaneously and intramuscular or intravascular delivery should be avoided. Instruct patients to use injection sites with an adequate skin and subcutaneous thickness to accommodate the length of the needle.
Patient Information
Patient Instructions for Use

Precautions with Alcohol
- Alcohol-Sumatriptan (injection) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- IMITREX®[1]
Look-Alike Drug Names
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "IMITREX- sumatriptan succinate injection".
- ↑ 2.0 2.1 "https://www.ismp.org". External link in
|title=(help)