Sapropterin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Turky Alkathery, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Sapropterin is a phenylalanine hydroxylase activator that is FDA approved for the treatment of high blood phenylalanine (Phe) levels in patients with hyperphenylalaninemia (HPA) due to tetrahydrobiopterin- (BH4-) responsive Phenylketonuria (PKU). Sapropterin is to be used in conjunction with a Phe-restricted diet.. Common adverse reactions include headache, rhinorrhea, pharyngolaryngeal pain, diarrhea, vomiting, cough, and nasal congestion.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indication
- Sapropterin is indicated to reduce blood phenylalanine (Phe) levels in patients with hyperphenylalaninemia (HPA) due to tetrahydrobiopterin- (BH4-) responsive Phenylketonuria (PKU). Sapropterin is to be used in conjunction with a Phe-restricted diet.
Dosage
- Patients 7 years and older: The recommended starting dose of Sapropterin is 10 to 20 mg/kg taken once daily.
- If a 10 mg/kg per day starting dose is used then response to therapy is determined by change in blood Phe following treatment with Sapropterin at 10 mg/kg per day for a period of up to 1 month. Blood Phe levels should be checked after 1 week of Sapropterin treatment and periodically for up to a month. If blood Phe does not decrease from baseline at 10 mg/kg per day, the dose may be increased to 20 mg/kg per day. Patients whose blood Phe does not decrease after 1 month of treatment at 20 mg/kg per day are non-responders and treatment with Sapropterin should be discontinued in these patients.
- If a 20 mg/kg per day starting dose is used then response to therapy is determined by change in blood Phe following treatment with Sapropterin at 20 mg/kg per day for a period of 1 month. Blood Phe levels should be checked after 1 week of Sapropterin treatment and periodically during the first month. Treatment should be discontinued in patients who do not respond to Sapropterin.
- Once responsiveness to Sapropterin has been established, the dosage may be adjusted within the range of 5 to 20 mg/kg per day according to response to therapy. Periodic blood Phe monitoring is recommended to assess blood Phe control.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Sapropterin in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Sapropterin in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Indication
- Sapropterin indicated to reduce blood phenylalanine (Phe) levels in patients with hyperphenylalaninemia (HPA)
Dosage
- Patients 1 month to 6 years: The recommended starting dose of Sapropterin is 10 mg/kg taken once daily.
- Patients 7 years and older: The recommended starting dose of Sapropterin is 10 to 20 mg/kg taken once daily.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Sapropterin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Sapropterin in pediatric patients.
Contraindications
None.
Warnings
Hypersensitivity Reactions Including Anaphylaxis
Sapropterin is not recommended in patients with a history of anaphylaxis to Sapropterin. Hypersensitivity reactions, including anaphylaxis and rash, have occurred. Signs of anaphylaxis include wheezing, dyspnea, coughing, hypotension, flushing, nausea, and rash. Discontinue treatment with Sapropterin in patients who experience anaphylaxis and initiate appropriate medical treatment. Continue dietary Phe restrictions in patients who experience anaphylaxis.
Gastritis
During clinical studies, gastritis was reported as a serious adverse reaction. Monitor patients for signs and symptoms of gastritis.
Hypophenylalaninemia
In clinical trials, some patients have experienced low blood Phe levels. Children younger than 7 years treated with Sapropterin doses of 20 mg/kg per day are at increased risk for low levels of blood Phe compared with patients 7 years and older.
Monitor Blood Phe Levels During Treatment
Treatment with Sapropterin should be directed by physicians knowledgeable in the management of PKU. Prolonged elevations in blood phe levels in patients with PKU can result in severe neurologic damage, including severe mental retardation, microcephaly, delayed speech, seizures, and behavioral abnormalities. Conversely, prolonged levels of blood Phe that are too low have been associated with catabolism and protein breakdown. Active management of dietary Phe intake while taking Sapropterin is required to ensure adequate Phe control and nutritional balance. Monitor blood Phe levels during treatment to ensure adequate blood Phe level control. Frequent blood monitoring is recommended in the pediatric population.
Identify Non-Responders to Sapropterin Treatment
Not all patients with PKU respond to treatment with Sapropterin. In two clinical trials at a dose of 20 mg/kg per day, 56% to 75% of pediatric PKU patients responded to treatment with Sapropterin, and in one clinical trial at a dose of 10 mg/kg per day, 20% of adult and pediatric PKU patients responded to treatment with Sapropterin.
Response to treatment cannot be pre-determined by laboratory testing (e.g., molecular testing), and can only be determined by a therapeutic trial of Sapropterin.
Treat All Patients with a Phe-restricted Diet
All patients with PKU who are being treated with Sapropterin should also be treated with a Phe-restricted diet.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reactions rates observed in the clinical trials of a drug cannot be directly compared to the rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
PKU Clinical Studies
The safety of Sapropterin was evaluated in 6 clinical studies in patients with PKU (aged 1 month to 50 years).
In Studies1-4 (controlled and uncontrolled studies), 579 patients with PKU aged 4 to 49 years received Sapropterin in doses ranging from 5 to 20 mg/kg per day for lengths of treatment ranging from 1 to 164 weeks. The patient population was evenly distributed in gender, and approximately 95% of patients were Caucasian. The most common adverse reactions (≥4% of patients) were headache, rhinorrhea, pharyngolaryngeal pain, diarrhea, vomiting, cough, and nasal congestion.
The data described in Table 3 reflect exposure of 74 patients with PKU to Sapropterin at doses of 10 to 20 mg/kg per day for 6 to 10 weeks in two double-blind, placebo-controlled clinical trials (Studies 2 and 4).
Table 3 enumerates adverse reactions occurring in at least 4% of patients treated with Sapropterin in the double-blind, placebo-controlled clinical trials described above.
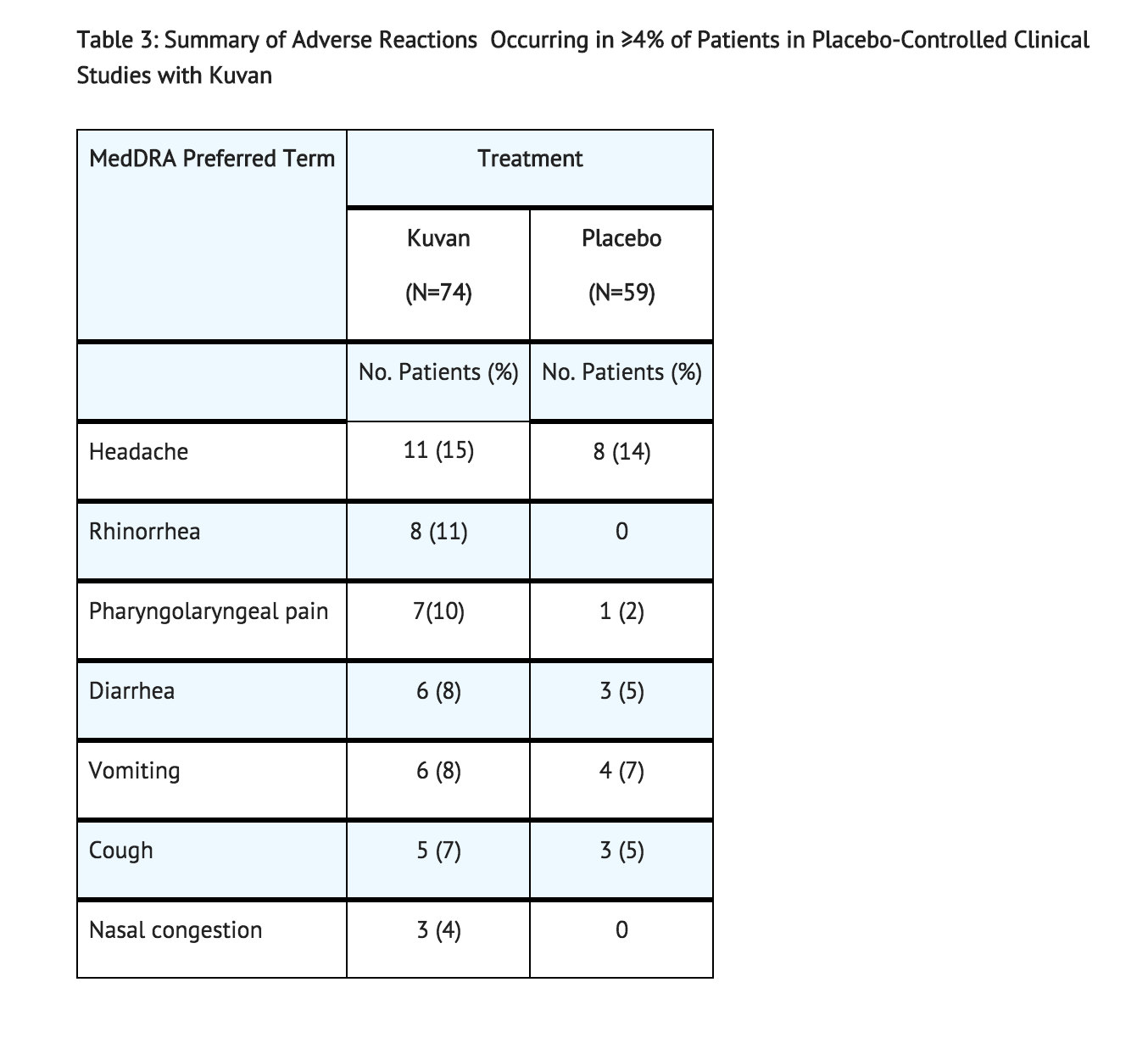
In open-label, uncontrolled clinical trials (Studies 1 and 3) all patients received Sapropterin in doses of 5 to 20 mg/kg per day, adverse reactions were similar in type and frequency to those reported in the double-blind, placebo-controlled clinical trials.
In Study 5, 65 pediatric patients with PKU aged 1 month to 6 years received Sapropterin 20 mg/kg per day for 6 months. Adverse reactions in these patients were similar in frequency and type as those seen in other Sapropterin clinical trials except for an increased incidence of low phe levels. Twenty-five percent (16 out of 65) of patients developed Phe levels below normal for age.
In Study 6, a long term, open-label, extension study of 111 patients aged 4 to 50 years, receiving Sapropterin in doses ranging from 5 to 20 mg/kg per day, adverse reactions were similar in type and frequency to those reported in the previous clinical studies. Fifty-five patients received Sapropterin both as dissolved and intact tablets. There were no notable differences in the incidence or severity of adverse reactions between the two methods of administration. The mean (± SD) exposure to sapropterin for the entire study population was 659 ± 221 days (maximum 953 days).
Safety Experience from Clinical Studies for Non-PKU Indications
Approximately 800 healthy volunteers and patients with disorders other than PKU, some of whom had underlying neurologic disorders or cardiovascular disease, have been administered a different formulation of the same active ingredient (sapropterin) in approximately 19 controlled and uncontrolled clinical trials. In these clinical trials, subjects were administered sapropterin at doses ranging from 1 to 100 mg/kg per day for lengths of exposure from 1 day to 2 years. Serious and severe adverse reactions (regardless of causality) during sapropterin administration were convulsions, exacerbation of convulsions, dizziness, gastrointestinal bleeding, post-procedural bleeding, headache, irritability, myocardial infarction, overstimulation, and respiratory failure. Common adverse reactions were headache, peripheral edema, arthralgia, polyuria, agitation, dizziness, nausea, pharyngitis, abdominal pain, upper abdominal pain, and upper respiratory tract infection.
Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Sapropterin. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish causal relationship to drug exposure.
In worldwide marketing experience, the most common adverse reactions due to Sapropterin are oropharyngeal pain, pharyngitis, esophageal pain, gastritis, dyspepsia, abdominal pain, nausea and vomiting. Hypersensitivity reactions including anaphylaxis and rash have been reported. Most hypersensitivity reactions occurred within several days of initiating treatment. Two cases of hyperactivity have been reported, including one case in a patient who received an accidental overdose of Sapropterin.
Drug Interactions
No drug interaction studies were performed.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C A patient registry has been established that collects data on women who are treated with Sapropterin during pregnancy. For more information regarding the registry program call 1-866-906-6100. Risk Summary
There are no adequate and well-controlled studies with Sapropterin in pregnant women. An embryo-fetal development study with sapropterin dihydrochloride in rats using oral doses up to 3 times the maximum recommended human dose (MRHD) given during the period of organogenesis showed no effects. In a rabbit study using oral administration of sapropterin dihydrochloride during the period of organogenesis, a rare defect, holoprosencephaly, was noted at 10 times the MRHD. Sapropterin should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Clinical Considerations
Disease-associated maternal and/or embryofetal risk
Available data from the Maternal Phenylketonuria Collaborative Study on 468 pregnancies and 331 live births in PKU‑affected women demonstrated that uncontrolled Phe levels above 600 µmol/L are associated with a very high incidence of neurological, cardiac, facial dysmorphism, and growth anomalies. Good dietary control of Phe levels during pregnancy is essential to reduce the incidence of Phe-induced teratogenic effects.
Animal Data
No effects on embryo-fetal development were observed in a reproduction study in rats using oral doses of up to 400 mg/kg per day sapropterin dihydrochloride (about 3 times the MRHD of 20 mg/kg per day, based on body surface area) administered during the period of organogenesis. However, in a rabbit reproduction study, oral administration of a maximum dose of 600 mg/kg per day (about 10 times the MRHD, based on body surface area) during the period of organogenesis was associated with a non-statistically significant increase in the incidence of holoprosencephaly in two high dose-treated litters (4 fetuses), compared to one control-treated litter (1 fetus).
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Sapropterin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Sapropterin during labor and delivery.
Nursing Mothers
It is not known whether Sapropterin is present in human milk. Sapropterin is present in the milk of intravenously, but not orally, treated lactating rats. The developmental and health benefits of human milk feeding should be considered along with the mother’s clinical need for Sapropterin and any potential adverse effects on the human milk-fed child from the drug or from the underlying maternal condition. Exercise caution when Sapropterin is administered to a nursing woman.
Pediatric Use
Pediatric patients with PKU, ages 1 month to 16 years, have been treated with Sapropterin in clinical trials.
The efficacy and safety of Sapropterin have not been established in neonates. The safety of Sapropterin has been established in children younger than 4 years in trials of 6 months duration and in children 4 years and older in trials of up to 3 years in length.
In children aged 1 month and older, the efficacy of Sapropterin has been demonstrated in trials of 6 weeks or less in duration.
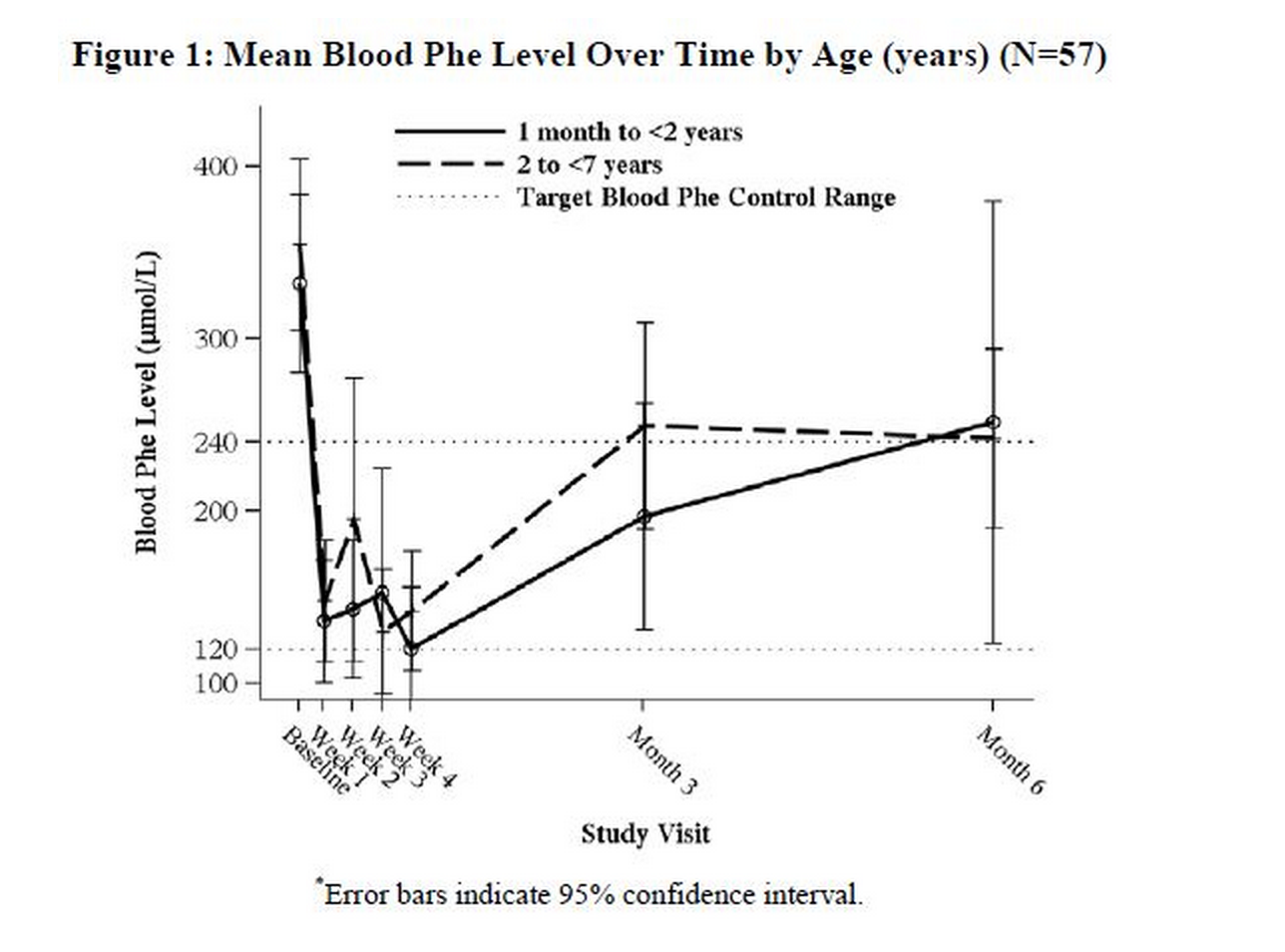
In a multicenter, open-label, single arm study, 57 patients aged 1 month to 6 years who were defined as Sapropterin responders after 4 weeks of Sapropterin treatment and Phe dietary restriction were treated for 6 months with Sapropterin at 20 mg/kg per day. The effectiveness of Sapropterin alone on reduction of blood Phe levels beyond 4 weeks could not be determined due to concurrent changes in dietary Phe intake during the study. Mean (±SD) blood Phe values over time for patients aged 1 month to <2 years and 2 to <7 years are shown in Figure 1.
Geriatic Use
Clinical studies of Sapropterin in patients with PKU did not include patients aged 65 years and older. It is not known whether these patients respond differently than younger patients.
Gender
There is no FDA guidance on the use of Sapropterin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Sapropterin with respect to specific racial populations.
Renal Impairment
Patients with renal impairment have not been evaluated in clinical trials. Monitor patients who have renal impairment carefully when they are receiving Sapropterin.
Hepatic Impairment
There is no FDA guidance on the use of Sapropterin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Sapropterin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Sapropterin in patients who are immunocompromised.
Administration and Monitoring
Administration
Sapropterin is available as tablets and as powder for oral solution. Sapropterin should be taken orally with a meal to increase absorption, preferably at the same time each day. A missed dose should be taken as soon as possible, but two doses should not be taken on the same day.
Instructions for Use
Sapropterin Tablets
Sapropterin tablets may be swallowed either as whole tablets or dissolved in 120 to 240 mL of water or apple juice and taken orally within 15 minutes of dissolution. It may take a few minutes for the tablets to dissolve. To make the tablets dissolve faster, tablets may be stirred or crushed. The tablets may not dissolve completely. Patients may see small pieces floating on top of the water or apple juice. This is normal and safe for patients to swallow. If after drinking the medicine patients still see pieces of the tablet in the container, more water or apple juice can be added to make sure all of the medicine is consumed. Sapropterin tablets may also be crushed and then mixed in a small amount of soft foods such as apple sauce or pudding.
Sapropterin Powder for Oral Solution
Sapropterin powder for oral solution should be dissolved in 120 to 240 mL of water or apple juice and taken orally within 30 minutes of dissolution. Sapropterin powder for oral solution may also be stirred in a small amount of soft foods such as apple sauce or pudding. Empty the contents of the packet(s) in water, apple juice, or a small amount of soft foods and mix thoroughly. The powder should dissolve completely.
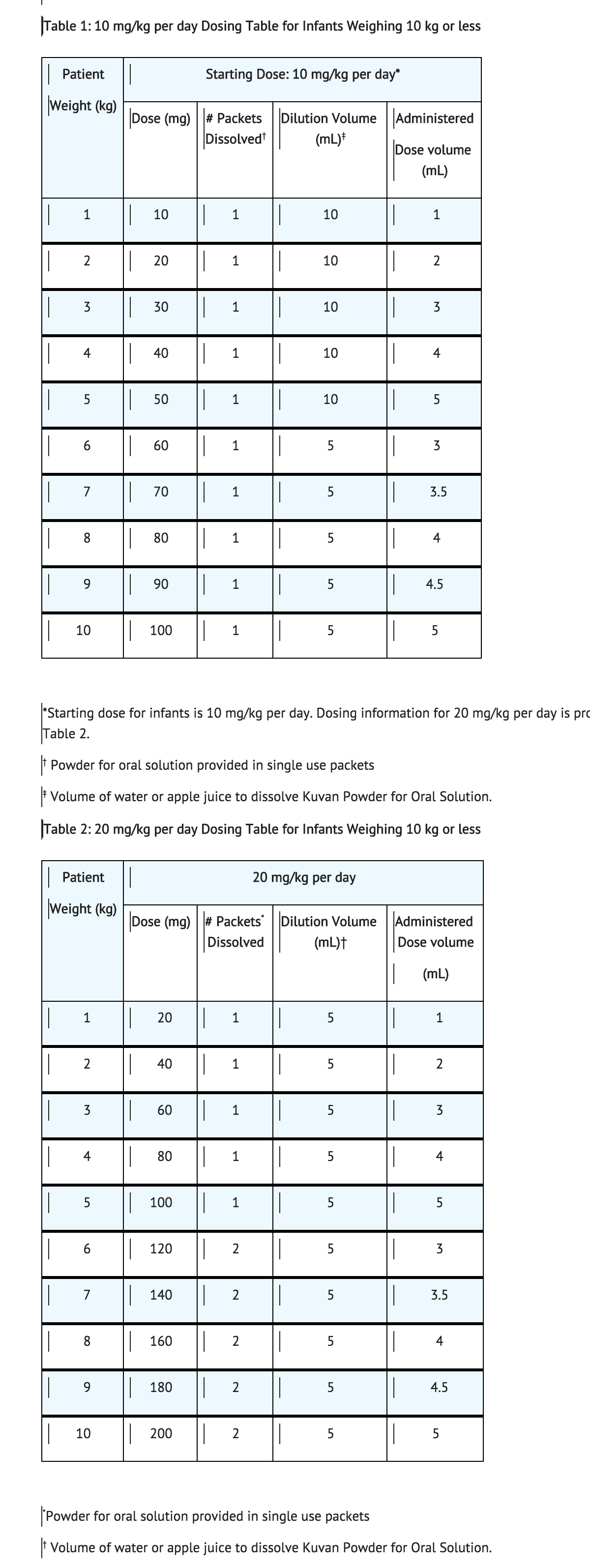
For infants weighing 10 kg or less, Sapropterin can be dissolved in as little as 5 mL of water or apple juice and a portion of this solution corresponding to a 10 mg/kg dose may be administered orally via an oral dosing syringe. Table 1 provides dosing information for infants at the recommended starting dose of 10 mg/kg per day. Refer to Table 2 for dosing information at 20 mg/kg per day if dosage adjustment is needed.
Monitoring
Monitor Patients with Hepatic Impairment
Patients with liver impairment have not been evaluated in clinical trials with Sapropterin. Monitor liver function tests in patients with liver impairment who are receiving Sapropterin because hepatic damage has been associated with impaired phe metabolism.
Monitor Patients when Co-administering Sapropterin and Medications Known to Inhibit Folate Metabolism
Co-administering Sapropterin with drugs known to affect folate metabolism (e.g., methotrexate) and their derivatives may require more frequent monitoring of blood phe levels because these drugs can decrease endogenous BH4 levels by inhibiting the enzyme dihydropteridine reductase (DHPR).
Monitor Patients for Hypotension when Co-administering Sapropterin and Drugs Known to Affect Nitric Oxide-Mediated Vasorelaxation
Monitor blood pressure when administering Sapropterin with drugs that affect nitric oxide‑mediated vasorelaxation (e.g., PDE-5 inhibitors such as sildenafil, vardenafil, or tadalafil, because both sapropterin dihydrochloride and PDE-5 inhibitors may induce vasorelaxation. The additive effect of sapropterin and PDE-5 inhibitor co-administration could lead to a reduction in blood pressure; however, the combined use of these medications has not been evaluated in humans. In animal studies, orally administered Sapropterin in combination with a PDE-5 inhibitor had no effect on blood pressure.
Monitor Patients when Co-administering Sapropterin and Levodopa
Caution should be used with the administration of Sapropterin to patients who are receiving levodopa. In a 10-year post-marketing safety surveillance program for a non-PKU indication using another formulation of the same active ingredient (sapropterin), 3 patients with underlying neurologic disorders experienced convulsions, exacerbation of convulsions, over-stimulation, or irritability during co-administration of levodopa and sapropterin. Monitor for change in neurologic status.
Monitor Patients for Hyperactivity
In the post-marketing safety surveillance program for PKU, 2 patients experienced hyperactivity with administration of Sapropterin. Monitor patients for hyperactivity.
IV Compatibility
There is limited information regarding the compatibility of Sapropterin and IV administrations.
Overdosage
Two unintentional overdosages with Sapropterin have been reported. One adult patient in a Sapropterin clinical trial received a single Sapropterin dose of 4,500 mg (36 mg/kg) instead of 2,600 mg (20 mg/kg). The patient reported mild headache and mild dizziness immediately after taking the dose; both symptoms resolved within 1 hour with no treatment intervention. There were no associated laboratory test abnormalities. The patient suspended therapy for 24 hours and then restarted Sapropterin with no reports of abnormal signs or symptoms. In postmarketing, one pediatric patient received Sapropterin doses of 45 mg/kg per day instead of 20 mg/kg per day. The patient reported hyperactivity that began at an unspecified time after overdose and resolved after the Sapropterin dose was reduced to 20 mg/kg per day.
In a clinical study to evaluate the effects of Sapropterin on cardiac repolarization, a single supra-therapeutic dose of 100 mg/kg (5 times the maximum recommended dose) was administered to 54 healthy adults. No serious adverse reactions were reported during the study. The only adverse reactions reported in more than 1 subject who received the supra-therapeutic dose were upper abdominal pain (6%) and dizziness (4%). A dose-dependent shortening of the QT interval was observed. Patients should be advised to notify their physicians in cases of overdose.
Pharmacology
| |
Sapropterin
| |
| Systematic (IUPAC) name | |
| (6R)-2-Amino-6-[(1R,2S)-1,2-dihydroxypropyl]-5,6,7,8-tetrahydropteridin-4(1H)-one | |
| Identifiers | |
| CAS number | |
| ATC code | A16 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 241.25 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | 4 hours (healthy adults) 6–7 hours (PKU patients) |
| Excretion | ? |
| Therapeutic considerations | |
| Licence data |
|
| Pregnancy cat. |
C(US) |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | Oral |
Mechanism of Action
Sapropterin is a synthetic form of BH4, the cofactor for the enzyme phenylalanine hydroxylase (PAH). PAH hydroxylates phe through an oxidative reaction to form tyrosine. In patients with PKU, PAH activity is absent or deficient. Treatment with BH4 can activate residual PAH enzyme activity, improve the normal oxidative metabolism of phe, and decrease Phe levels in some patients.
Structure
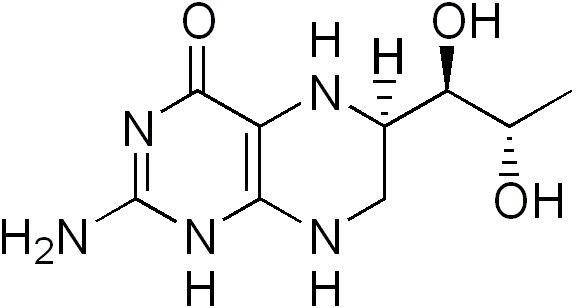
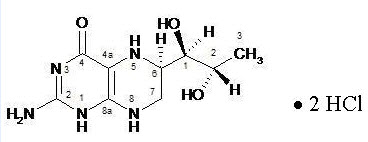
Sapropterin dihydrochloride is an orally administered Phenylalanine Hydroxylase activator (or PAH activator). Sapropterin dihydrochloride, the active pharmaceutical ingredient in Sapropterin, is a synthetic preparation of the dihydrochloride salt of naturally occurring tetrahydrobiopterin (BH4). Sapropterin dihydrochloride is an off-white to light yellow crystals or crystalline powder.
The chemical name of sapropterin dihydrochloride is (6R)-2-amino-6-[(1R,2S)-1,2-dihydroxypropyl]-5,6,7,8-tetrahydro-4(1H)-pteridinone dihydrochloride and the molecular formula is C9H15N5O3·2HCl with a molecular weight of 314.17.
Sapropterin dihydrochloride has the following structural formula:
Sapropterin is supplied as tablets and powder for oral solution containing 100 mg of sapropterin dihydrochloride (equivalent to 76.8 mg of sapropterin base).
Tablets are round, off-white to light yellow, mottled, and debossed with “177”. Each tablet contains the following inactive ingredients: ascorbic acid (USP), crospovidone (NF), dibasic calcium phosphate (USP), D-mannitol (USP), riboflavin (USP), and sodium stearyl fumarate (NF).
Sapropterin powder for oral solution is off-white to yellow in color. Each unit dose packet contains the following inactive ingredients: ascorbic acid (USP), D-mannitol (USP), potassium citrate (USP), and sucralose (NF).
Pharmacodynamics
In PKU patients who are responsive to BH4 treatment, blood phe levels decrease within 24 hours after a single administration of sapropterin dihydrochloride, although maximal effect on Phe level may take up to a month, depending on the patient. A single daily dose of Sapropterin is adequate to maintain stable blood Phe levels over a 24-hour period. Twelve patients with blood Phe levels ranging from 516 to 986 μmol/L (mean 747 ± 153 μmol/L) were assessed with 24‑hour blood Phe level monitoring following a daily morning dose of 10 mg/kg per day. The blood Phe level remained stable during a 24‑hour observation period. No substantial increases in blood Phe levels were observed following food intake throughout the 24-hour period.
Sapropterin dose-response relationship was studied in an open-label, forced titration study at doses of 5 mg/kg per day, then 20 mg/kg per day, and then 10 mg/kg per day (Study 3). Individual blood phe levels were highly variable among patients. The mean blood Phe level observed at the end of each 2-week dosing period decreased as the dose of sapropterin dihydrochloride increased, demonstrating an inverse relationship between the dose of sapropterin dihydrochloride and mean blood Phe levels.
Effects of Sapropterin on the QTc interval
A thorough QTc study was performed in 56 healthy adults. This randomized, placebo and active controlled crossover study was conducted to determine if a single supra-therapeutic (100 mg/kg) of Sapropterin, or a single therapeutic dose (20 mg/kg) of Sapropterin had an effect on cardiac repolarization. In this study, Sapropterin was administered after dissolving tablets in water under fed condition. This study demonstrated a dose-dependent shortening of the QT interval. The maximum placebo-subtracted mean change from baseline of the QTc interval was -3.69 and -8.32 ms (lower bound of 90% CI: -5.3 and -10.6 ms) at 20 and 100 mg/kg, respectively.
Pharmacokinetics
Studies in healthy volunteers have shown comparable absorption of sapropterin when tablets are dissolved in water or orange juice and taken under fasted conditions. Administration of dissolved tablets after a high-fat/high-calorie meal resulted in mean increases in Cmax of 84% and AUC of 87% (dissolved in water). However, there was extensive variability in individual subject values for Cmax and AUC across the different modes of administration and meal conditions. In the clinical trials of Sapropterin, drug was administered in the morning as a dissolved tablet without regard to meals. The mean elimination half-life in PKU patients was approximately 6.7 hours (range 3.9 to 17 hr), comparable with values seen in healthy subjects (range 3.0 to 5.3 hr).
A study in healthy adults with 10 mg/kg of Sapropterin demonstrated the absorption via intact tablet administration was 40% greater than via dissolved tablet administration under fasted conditions based on AUC0-t. The administration of intact tablets under fed conditions resulted in an approximately 43% increase in the extent of absorption compared to fasted conditions based on AUC0-t.
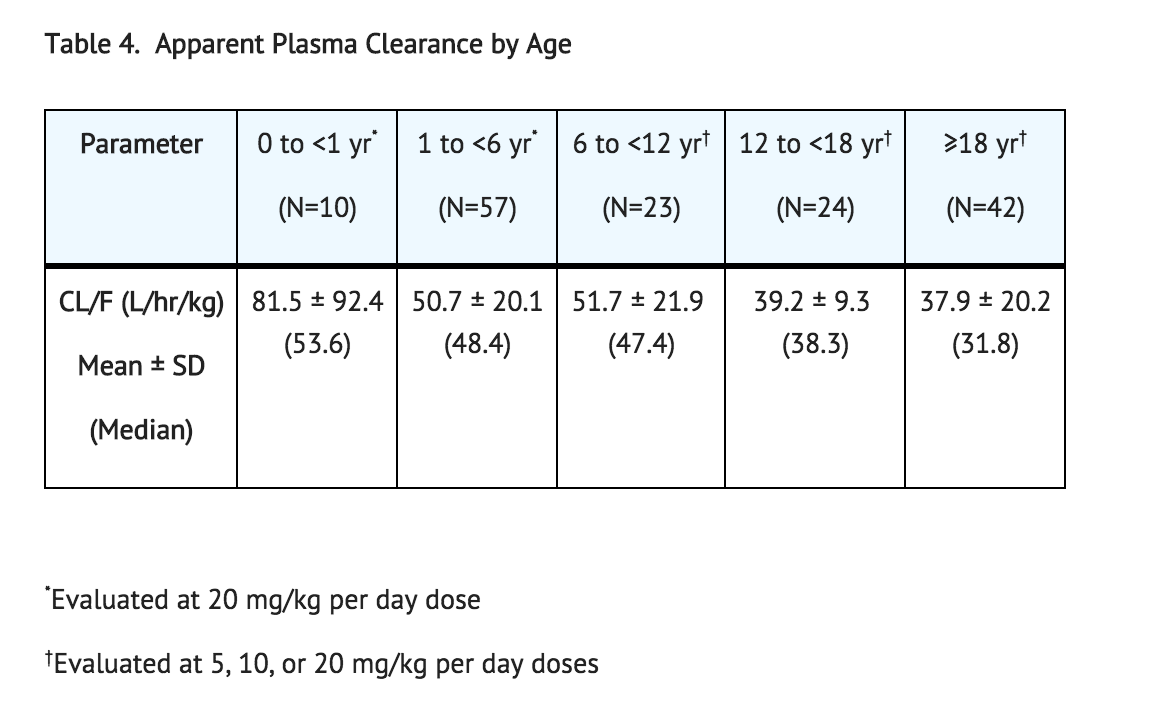
Population pharmacokinetic analysis of sapropterin including patients from 1 month to 49 years of age showed that body weight is the only covariate substantially affecting clearance or distribution volume (see Table 4). Pharmacokinetics in patients >49 years of age have not been studied.
Metabolism
Sapropterin is a synthetic form of tetrahydrobiopterin (BH4) and is expected to be metabolized and recycled by the same endogenous enzymes. In vivo endogenous BH4 is converted to quinoid dihydrobiopterin and is metabolized to dihydrobiopterin and biopterin. The enzymes dihydrofolate reductase and dihydropteridine reductase are responsible for the metabolism and recycling of BH4.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
A 2-year carcinogenicity study was conducted in F-344 rats, and a 78-week carcinogenicity study was conducted in CD-1 mice. In the 104-week oral carcinogenicity study in rats, sapropterin dihydrochloride doses of 25, 80, and 250 mg/kg per day (0.2, 0.7, and 2 times the maximum recommended human dose of 20 mg/kg per day, respectively, based on body surface area) were used. In the 78-week oral carcinogenicity study in mice, sapropterin dihydrochloride doses of 25, 80, and 250 mg/kg per day (0.1, 0.3, and 2 times the recommended human dose, respectively, based on body surface area) were used. In the 2‑year rat carcinogenicity study, there was a statistically significant increase in the incidence of benign adrenal pheochromocytoma in male rats treated with the 250 mg/kg per day (about 2 times the maximum recommended human dose, based on body surface area) dose, as compared to vehicle treated rats. The mouse carcinogenicity study showed no evidence of a carcinogenic effect, but the study was not ideal due to its duration of 78 instead of 104 weeks.
Sapropterin dihydrochloride was genotoxic in the in vitro Ames test at concentrations of 625 µg (TA98) and 5000 µg (TA100) per plate, without metabolic activation. However, no genotoxicity was observed in the in vitro Ames test with metabolic activation. Sapropterin dihydrochloride was genotoxic in the in vitro chromosomal aberration assay in Chinese hamster lung cells at concentrations of 0.25 and 0.5 mM. Sapropterin dihydrochloride was not mutagenic in the in vivo micronucleus assay in mice at doses up to 2000 mg/kg per day (about 8 times the maximum recommended human dose of 20 mg/kg per day, based on body surface area). Sapropterin dihydrochloride, at oral doses up to 400 mg/kg per day (about 3 times the maximum recommended human dose, based on body surface area) was found to have no effect on fertility and reproductive function of male and female rats.
Clinical Studies
The efficacy of Sapropterin was evaluated in five clinical studies in patients with PKU.
Study 1 was a multicenter, open-label, uncontrolled clinical trial of 489 patients with PKU, ages 8 to 48 years (mean 22 years), who had baseline blood phe levels ≥ 450 μmol/L and who were not on Phe-restricted diets. All patients received treatment with Sapropterin 10 mg/kg per day for 8 days. For the purposes of this study, response to Sapropterin treatment was defined as a ≥ 30% decrease in blood Phe from baseline. At Day 8, 96 patients (20%) were identified as responders.
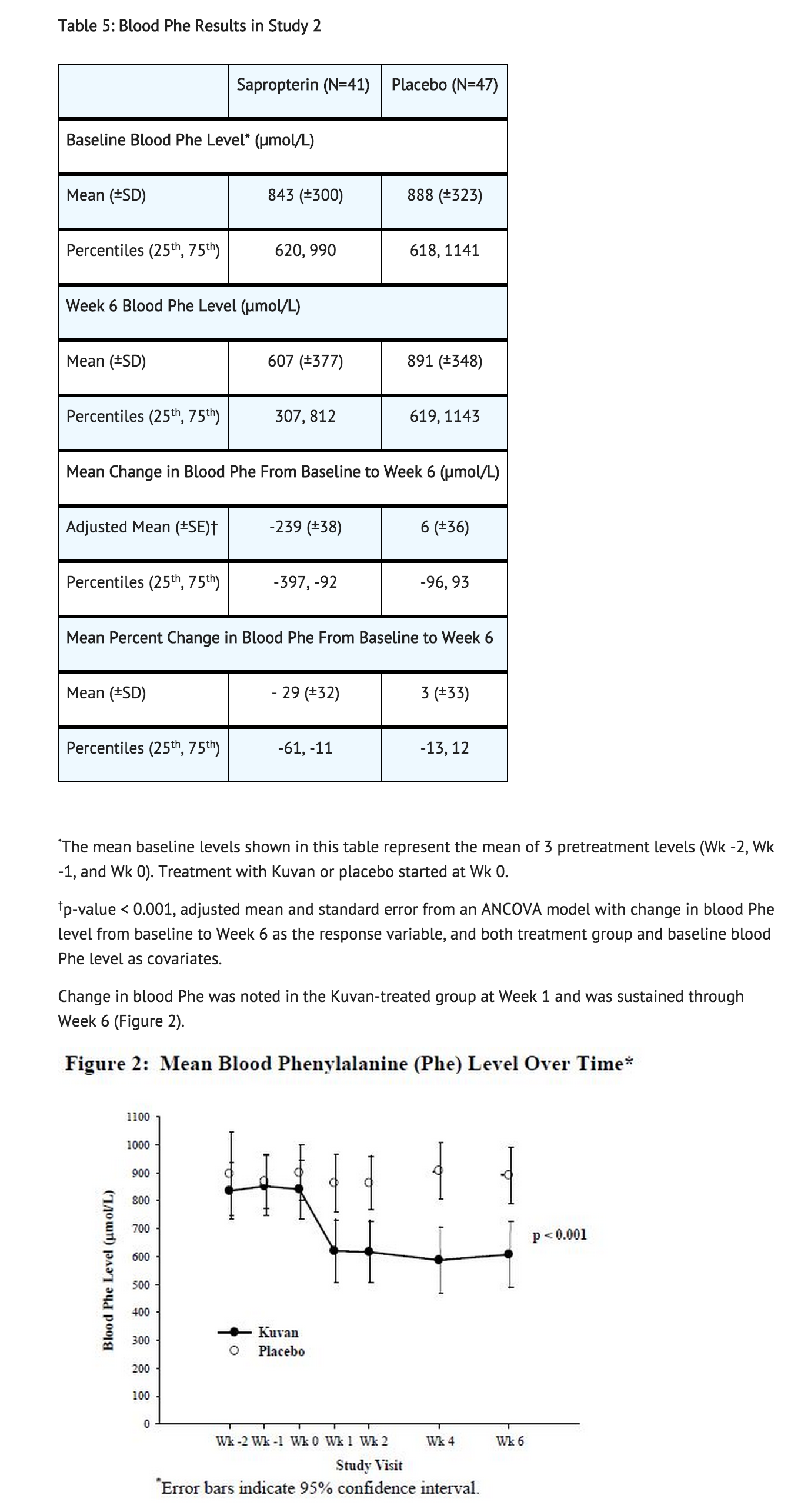
Study 2 was a multicenter, double-blind, placebo-controlled study of 88 patients with PKU who responded to Sapropterin in Study 1. After a washout period from Study 1, patients were randomized equally to either Sapropterin 10 mg/kg per day (N=41) or placebo (N=47) for 6 weeks. Efficacy was assessed by the mean change in blood Phe level from baseline to Week 6 in the Sapropterin-treated group as compared to the mean change in the placebo group.
The results showed that at baseline, the mean (±SD) blood phe level was 843 (±300) μmol/L in the Sapropterin-treated group and 888 (±323) μmol/L in the placebo group. At Week 6, the Sapropterin treated group had a mean (±SD) blood Phe level of 607 (±377) μmol/L, and the placebo group had a mean blood Phe level of 891 (±348) μmol/L. At Week 6, the Sapropterin- and placebo treated groups had mean changes in blood Phe level of –239 and 6 μmol/L, respectively (mean percent changes of –29% (±32) and 3% (±33), respectively). The difference between the groups was statistically significant (p < 0.001) (Table 5).
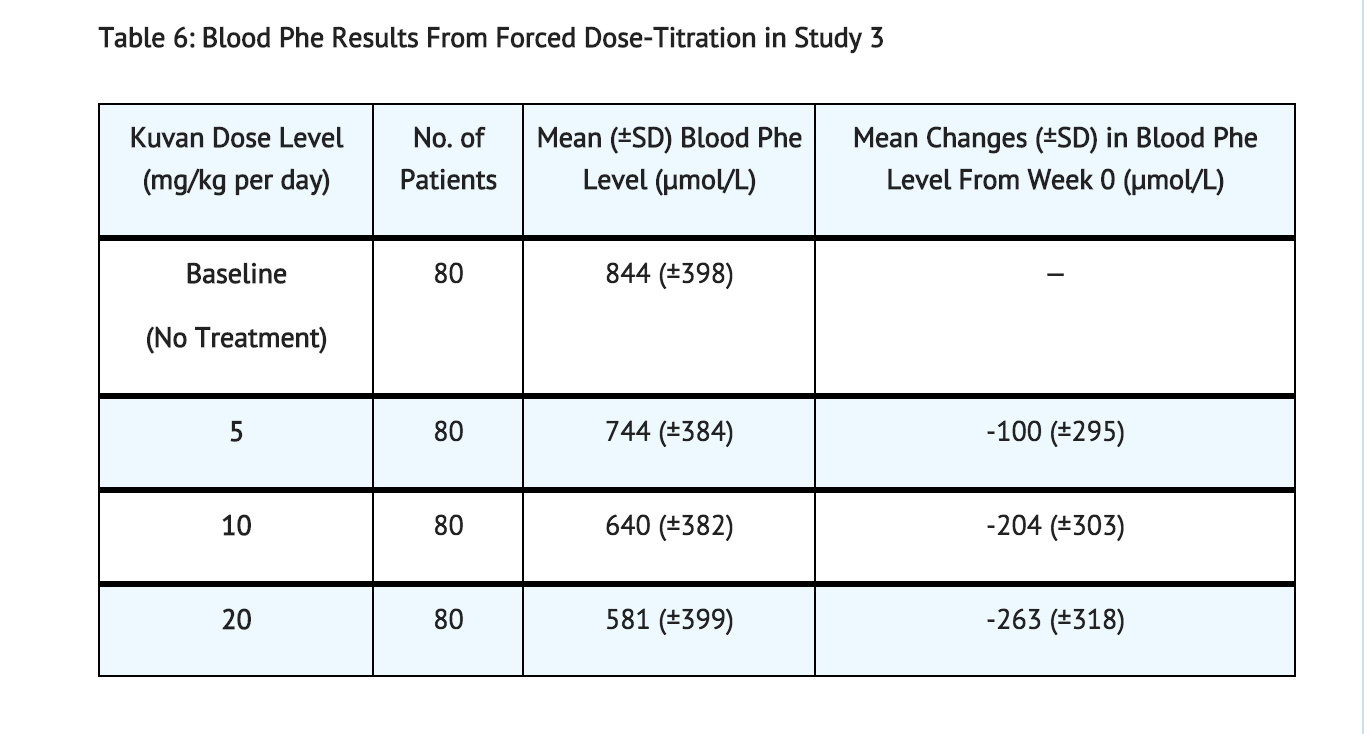
Study 3 was a multicenter, open-label, extension study in which 80 patients who responded to Sapropterin treatment in Study 1 and completed Study 2 underwent 6 weeks of forced dose-titration with 3 different doses of Sapropterin. Treatments consisted of 3 consecutive 2-week courses of Sapropterin at doses of 5, then 20, and then 10 mg/kg per day. Blood phe level was monitored after 2 weeks of treatment at each dose level. At baseline, mean (±SD) blood Phe was 844 (±398) μmol/L. At the end of treatment with 5, 10, and 20 mg/kg per day, mean (±SD) blood Phe levels were 744 (±384) μmol/L, 640 (±382) μmol/L, and 581 (±399) μmol/L, respectively (Table 6).
Study 4 was a multicenter study of 90 pediatric patients with PKU, ages 4 to 12 years, who were on Phe‑restricted diets and who had blood phe levels ≤480 μmol/L at screening. All patients were treated with open-label Sapropterin 20 mg/kg per day for 8 days. Response to Sapropterin was defined as a ≥30% decrease in blood Phe from baseline at Day 8. At Day 8, 50 patients (56%) had a ≥30% decrease in blood Phe.
Study 5 was an open label, single arm, multicenter trial in 93 pediatric patients with PKU, aged 1 month to 6 years, who had Phe levels greater than or equal to 360 μmol/L at screening. All patients were treated with Sapropterin at 20 mg/kg per day and maintained on a Phe-restricted diet. At Week 4, 57 patients (61%) were identified as responders (defined as ≥ 30% decreased in blood Phe from baseline) (see Figure 1 section 8.4).
How Supplied
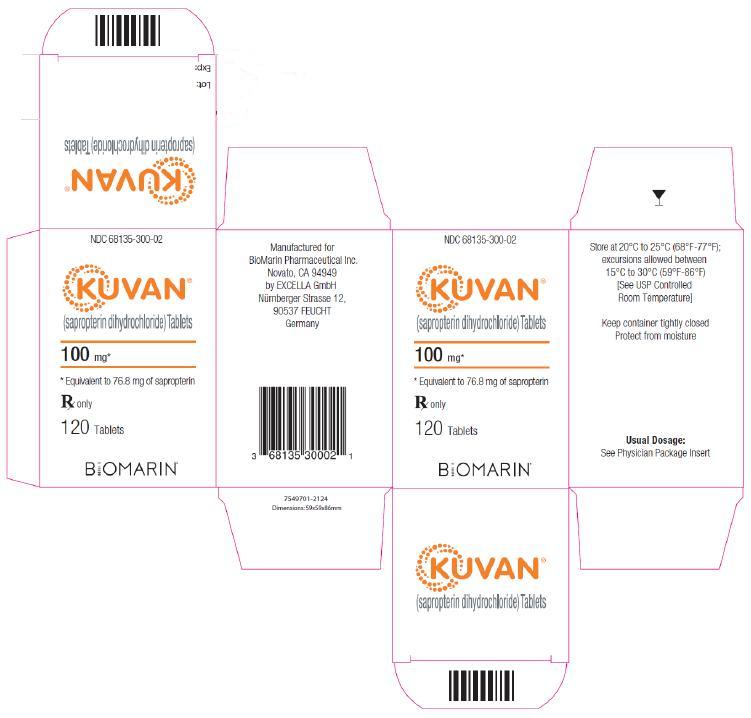
Sapropterin tablets, 100 mg, are round, off-white to light yellow, mottled, and debossed with “177”. The tablets are supplied as follows:
NDC 68135-300-02 Bottle of 120 tablets
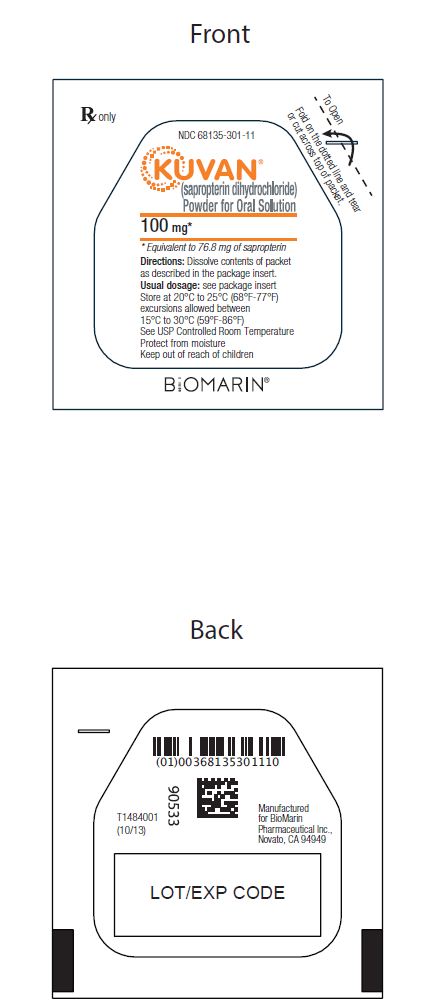
Sapropterin powder for oral solution, 100 mg, is an off-white to yellow powder. Sapropterin powder is packaged in unit dose packets as follows:
NDC 68135-301-22 Carton of 30 unit dose packets
NDC 68135-301-11 Single unit dose packet
Storage
Store Sapropterin tablets at 20ºC to 25ºC (68ºF to 77ºF); excursions allowed between 15ºC to 30ºC (59ºF to 86ºF) [see USP Controlled Room Temperature]. Keep container tightly closed. Protect from moisture.
Store Sapropterin powder for oral solution at 20ºC to 25ºC (68ºF to 77ºF); excursions allowed between 15ºC to 30ºC (59ºF to 86ºF) [see USP Controlled Room Temperature]. Protect from moisture.
Images
Drug Images
{{#ask: Page Name::Sapropterin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Sapropterin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Patients should be advised of the following information before beginning treatment with Kuvan:
- Advise patients that Kuvan may cause low blood Phe levels.
- Advise patients that children younger than 7 years treated with Kuvan doses of 20 mg/kg per day are at increased risk for low levels of blood phe compared with children 7 years and older. Blood phe levels that are too low for prolonged periods of time may be associated with catabolism and protein breakdown.
- Advise patients that Kuvan is to be used in conjunction with a Phe-restricted diet.
- Advise patients that not all patients with PKU respond to treatment with Kuvan and that response to Kuvan can only be determined by a therapeutic trial.
- Advise patients that they must be evaluated for changes in blood Phe after being treated with Kuvan at the recommended dose(s) for age to determine if they are a responder and that blood Phe levels and dietary Phe intake should be measured frequently during the first month.
- Advise patients that they should have frequent blood Phe measurements and nutritional counseling with their physician and other members of the health care team knowledgeable in the management of PKU to ensure maintenance of blood Phe levels in the desirable range.
- Advise patients not to modify their existing dietary Phe intake during the evaluation period in order to get an accurate assessment of the effect of Kuvan on blood Phe levels.
- Advise patients not to continue treatment with Kuvan if they are determined to be a non-responder during the evaluation period.
- Advise patients that reduction of blood Phe levels through dietary control is an important determinant of long-term neurologic outcome in PKU patients. Advise patients that the effect of Kuvan on long-term neurologic function in patients with PKU has not been assessed.
- Advise patients that Kuvan may cause hypersensitivity reactions including anaphylaxis and rash.
- Advise patients to notify their physician for symptoms of severe gastritis.
- Advise patients that blood Phe levels that are too high for prolonged periods of time can result in neurologic impairment.
- Advise patients that adequate blood Phe control needs to be maintained to avoid blood Phe levels that are too high or too low.
- Advise patients that to ensure maintenance of adequate blood Phe control, close monitoring is recommended and that the dose of Kuvan should be adjusted if necessary.
- Advise patients with hepatic impairment , and patients who are taking Kuvan in combination with drugs that inhibit folate metabolism, drugs that affect nitric oxide-mediated vasorelaxation, or levodopa that they may require additional clinical monitoring while taking Kuvan.
- Advise patients that Kuvan may cause hyperactivity.
- Advise patients that BioMarin has a product registry for PKU patients to collect data on women who become pregnant while receiving Kuvan treatment.
Precautions with Alcohol
Alcohol-Sapropterin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- KUVAN ®[1]
Look-Alike Drug Names
There is limited information regarding Sapropterin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "KUVAN- sapropterin dihydrochloride tablet KUVAN- sapropterin dihydrochloride powder, for solution". line feed character in
|title=at position 43 (help)