Rimexolone
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Rimexolone is a glucocorticoid that is FDA approved for the treatment of post-operative inflammation and anterior uveitis. Common adverse reactions include hypotension, erythema, pruritus, taste sense altered, headache, blurred vision, discharge from eye, pain in eye, pharyngitis, and rhinitis.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Post-Operative Inflammation
- Apply one - two drops of VEXOL® 1% Ophthalmic Suspension into the conjunctival sac of the affected eye four times daily beginning 24 hours after surgery and continuing throughout the first 2 weeks of the postoperative period.
Anterior Uveitis
- Apply one - two drops of VEXOL® 1% Ophthalmic Suspension into the conjunctival sac of the affected eye every hour during waking hours for the first week, one drop every two hours during waking hours of the second week, and then taper until uveitis is resolved.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Rimexolone in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Rimexolone in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Rimexolone in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Rimexolone in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Rimexolone in pediatric patients.
Contraindications
- VEXOL® 1% (rimexolone ophthalmic suspension) is contraindicated in epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, varicella, and most other viral diseases of the cornea and conjunctiva; mycobacterial infection of the eye; fungal diseases of the eye; acute purulent untreated infections which, like other diseases caused by microorganisms, may be masked or enhanced by the presence of the steroid; and in those persons with hypersensitivity to any component of the formulation.
Warnings
- For topical ophthalmic use only. Not for injection. Use in the treatment of herpes simplex infection requires great caution and frequent slit-lamp examinations. Prolonged use may result in ocular hypertension/glaucoma, damage to the optic nerve, defects in visual acuity and visual fields, and posterior subcapsular cataract formation.
- Prolonged use may also result in secondary ocular infections due to suppression of host response.
- Acute purulent infections of the eye may be masked or exacerbated by the presence of corticosteroid medication. In those diseases causing thinning of the cornea or sclera, perforation has been known to occur with topical steroids. It is advisable that the intraocular pressure be checked frequently.
Precautions
- Fungal infections of the cornea are particularly prone to develop coincidentally with long-term local steroid application. Fungal invasion must be considered in any persistent corneal ulceration where a steroid has been or is in use.
- For ophthalmic use only. The initial prescription and renewal of the medication order beyond 14 days should be made by a physician only after examination of the patient with the aid of magnification, such as slit lamp biomicroscopy and where appropriate, fluorescein staining. If signs and symptoms fail to improve after two days, the patient should be reevaluated.
- If this product is used for 10 days or longer, intraocular pressure should be monitored even though it may be difficult in children and uncooperative patients.
Adverse Reactions
Clinical Trials Experience
- Reactions associated with ophthalmic steroids include elevated intraocular pressure, which may be associated with optic nerve damage, visual acuity and field defects, posterior subcapsular cataract formation, secondary ocular infection from pathogens including herpes simplex, and perforation of the globe where there is thinning of the cornea or sclera.
- Ocular adverse reactions occurring in 1 - 5% of patients in clinical studies of VEXOL® 1% (rimexolone ophthalmic suspension) included blurred vision, discharge, discomfort, ocular pain, increased intraocular pressure, foreign body sensation, hyperemia and pruritus.
- Other ocular adverse reactions occurring in less than 1% of patients included sticky sensation, increased fibrin, dry eye, conjunctival edema, corneal staining, keratitis, tearing, photophobia, edema, irritation, corneal ulcer, browache, lid margin crusting, corneal edema, infiltrate, and corneal erosion.
- Non-ocular adverse reactions occurred in less than 2% of patients. These included headache, hypotension, rhinitis, pharyngitis, and taste perversion.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Rimexolone in the drug label.
Drug Interactions
There is limited information regarding Rimexolone Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Pregnancy Category C
- Rimexolone has been shown to be teratogenic and embryotoxic in rabbits following subcutaneous administration at the lowest dose tested (0.5 mg/kg/day, approximately 2 times the recommended human ophthalmic dose). Corticosteroids are recognized to cause fetal resorptions and malformations in animals. There are no adequate and well-controlled studies in pregnant women. VEXOL® 1% (rimexolone ophthalmic suspension) should be used in pregnant women only if the potential benefit to the mother justifies the potential risk to the fetus.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Rimexolone in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Rimexolone during labor and delivery.
Nursing Mothers
- It is not known whether topical ophthalmic administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in human breast milk. Nevertheless, caution should be exercised when topical corticosteroids are administered to a nursing woman; a decision should be made whether to discontinue nursing or discontinue therapy, taking into consideration the importance of the drug to the mother.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
- No overall differences in safety or effectiveness have been observed between elderly and younger patients.
Gender
There is no FDA guidance on the use of Rimexolone with respect to specific gender populations.
Race
There is no FDA guidance on the use of Rimexolone with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Rimexolone in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Rimexolone in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Rimexolone in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Rimexolone in patients who are immunocompromised.
Administration and Monitoring
Administration
- Topical
Monitoring
There is limited information regarding Monitoring of Rimexolone in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Rimexolone in the drug label.
Overdosage
Chronic Overdose
There is limited information regarding Chronic Overdose of Rimexolone in the drug label.
Pharmacology

| |
Rimexolone
| |
| Systematic (IUPAC) name | |
| (8S,9S,10R,11S,13S,14S,16R,17S)-11-Hydroxy-10,13,16,17-tetramethyl-17-propanoyl-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-3-one | |
| Identifiers | |
| CAS number | |
| ATC code | H02 S01BA13 (WHO) |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 370.525 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
C(US) |
| Legal status | |
| Routes | ocular |
Mechanism of Action
- Corticosteroids suppress the inflammatory response to a variety of inciting agents of a mechanical, chemical, or immunological nature. They inhibit edema, cellular infiltration, capillary dilatation, fibroblastic proliferation, deposition of collagen and scar formation associated with inflammation. Placebo-controlled clinical studies demonstrated that VEXOL® 1% Ophthalmic Suspension is efficacious for the treatment of anterior chamber inflammation following cataract surgery.
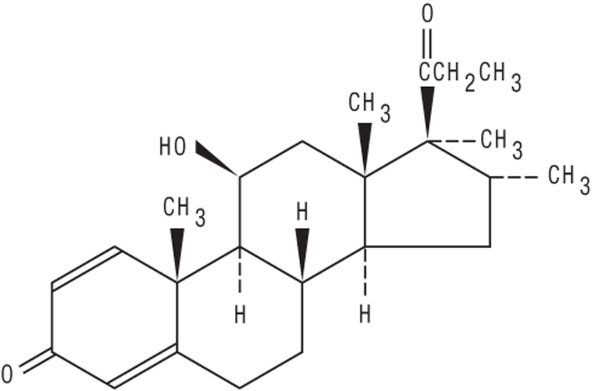
Structure
- VEXOL® 1% Ophthalmic Suspension is a sterile, multi-dose topical ophthalmic suspension containing the corticosteroid, rimexolone. Rimexolone is a white, water-insoluble powder with an empirical formula of C24H34O3 and a molecular weight of 370.53. Its chemical name is 11β-Hydroxy-16α,17α-dimethyl-17-propio nylandrosta-1,4-diene-3-one.The chemical structure of rimexolone is presented below:
- Each mL Contains: Active ingredient: rimexolone 10 mg (1%). Preservative: benzalkonium chloride 0.01%. Inactive ingredients: carbomer 974P, polysorbate 80, sodium chloride, edetate disodium, sodium hydroxide and/or hydrochloric acid (to adjust pH) and purified water.
- The pH of the suspension is 6.0 to 8.0 and the tonicity is 260 to 320 mOsmol/kg.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Rimexolone in the drug label.
Pharmacokinetics
- In two controlled clinical trials, VEXOL® 1% Ophthalmic Suspension demonstrated clinical equivalence to 1% prednisolone acetate in reducing uveitic inflammation. In a controlled 6-week study of steroid responsive subjects, the time to raise intraocular pressure was similar for VEXOL® 1% Ophthalmic Suspension and 0.1% fluorometholone given four times daily.
- As with other topically administered ophthalmic drugs, VEXOL® 1% (rimexolone ophthalmic suspension) is absorbed systemically. Studies in normal volunteers dosed bilaterally once every hour during waking hours for one week have demonstrated serum concentrations ranging from less than 80 pg/mL to 470 pg/mL. The mean serum concentrations were approximately 130 pg/mL. Serum concentrations were at or near steady state after 5 to 7 hourly doses. After decreasing the dosing frequency to once every two hours while awake during the second week of administration, mean serum concentrations were approximately 100 pg/mL.
- The serum half-life of rimexolone could not be reliably estimated due to the large number of samples below the quantitation limit of the assay (80 pg/mL). However, based on the time required to reach steady-state, the half-life appears to be short (1 - 2 hours).
- Based upon in vivo and in vitro preclinical metabolism studies, and on in vitro results with human liver preparations, rimexolone undergoes extensive metabolism. Following IV administration of radio-labeled rimexolone to rats, greater than 80% of the dose is excreted via the feces as rimexolone and metabolites. Metabolites have been shown to be less active than parent drug, or inactive in human glucocorticoid receptor binding assays.
Nonclinical Toxicology
- Rimexolone has been shown to be non-mutagenic in a battery of in vitro and in vivo mutagenicity assays.
- Fertility and reproductive capability were not impaired in a study in rats with plasma levels (42 ng/mL) approximately 200 times those obtained in clinical studies after topical administration (<0.2 ng/mL). Long-term studies have not been conducted in animals or humans to evaluate the carcinogenic potential of rimexolone.
Clinical Studies
There is limited information regarding Clinical Studies of Rimexolone in the drug label.
How Supplied
- 5 mL and 10 mL in plastic DROP-TAINER® dispensers. VEXOL® 1% Ophthalmic Suspension is supplied in natural (clear) low density polyethylene (LDPE) bottles, with a natural LDPE dispensing plug and pink polypropylene closures. Fill volumes are 5 mL in an 8 mL bottle and 10 mL in a 10 mL bottle.
- 5 mL: NDC 0065-0627-07
- 10 mL: NDC 0065-0627-03
- Storage: Store upright between 2°-25°C (36°-77°F).
- Do not freeze.
- Shake well before using.
Storage
There is limited information regarding Rimexolone Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Rimexolone |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Rimexolone |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Do not touch dropper tip to any surface, as this may contaminate the suspension.
Precautions with Alcohol
- Alcohol-Rimexolone interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- VEXOL®[1]
Look-Alike Drug Names
- Vosol® — Vexol®[2]
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "VEXOL rimexolone suspension".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Rimexolone
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Rimexolone |Label Name=Rimexolone02.png
}}
{{#subobject:
|Label Page=Rimexolone |Label Name=Rimexolone03.png
}}