Transplant rejection
| Transplant rejection | |
 | |
|---|---|
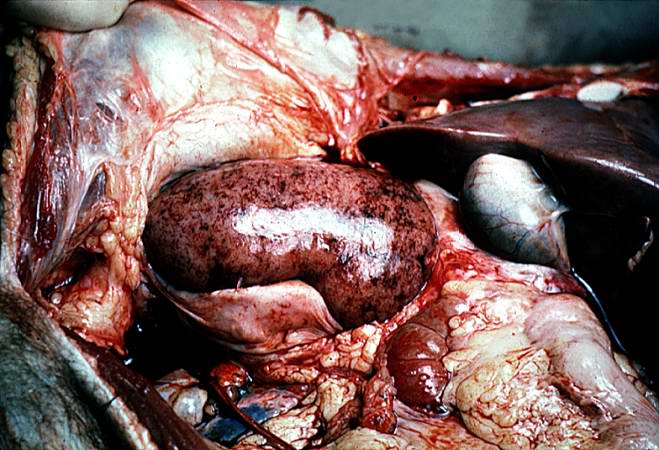
| A gross photograph of a kidney with acute rejection from an autopsy case. Note that the kidney is swollen (edema and inflammation) and there are areas of hemorrhage throughout the kidney. Image courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology |
Template:Search infobox Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Transplant rejection occurs when the immune system of the recipient of a transplant attacks the transplanted organ or tissue. This is because a normal healthy human immune system can distinguish foreign tissues and attempt to destroy them, just as it attempts to destroy infective organisms such as bacteria and viruses.
Types of rejection
Hyperacute rejection
Hyperacute rejection is a complement-mediated response in recipients with pre-existing antibodies to the donor (for example, ABO blood type antibodies). Hyperacute rejection occurs within minutes and the transplant must be immediately removed to prevent a severe systemic inflammatory response. Rapid agglutination of the blood occurs. This is a particular risk in kidney transplants, and so a prospective cytotoxic crossmatch is performed prior to kidney transplantation to ensure that antibodies to the donor are not present. For other organs, hyperacute rejection is prevented by transplanting only ABO-compatible grafts. Hyperacute rejection is the likely outcome of xenotransplanted organs.
Acute rejection
Acute rejection is generally acknowledged to be mediated by T cell responses to proteins from the donor organ which differ from those found in the recipient. Unlike antibody-mediated hyperacute rejection, development of T-cell responses first occurs several days after a transplant if the patient is not taking immunosuppressant drugs. Since the development of powerful immunosuppressive drugs such as cyclosporin, tacrolimus and rapamycin, the incidence of acute rejection has been greatly decreased, however, organ transplant recipients can develop acute rejection episodes months to years after transplantation. Acute rejection episodes can destroy the transplant if it is not recognized and treated appropriately. Episodes occur in around 60-75% of first kidney transplants, and 50 to 60% of liver transplants. A single episode is not a cause for concern if recognised and treated promptly and rarely leads to organ failure, but recurrent episodes are associated with chronic rejection of grafts. The bulk of the immune system response is to the Major Histocompatibility Complex (MHC) proteins. MHC proteins are involved in the presentation of foreign antigens to T-cells, and receptors on the surface of the T-cell (TCR) are uniquely suited to recognition of proteins of this type. MHC are highly variable between individuals, and therefore the T-cells from the host recognize the foreign MHC with a very high frequency leading to powerful immune responses that cause rejection of transplanted tissue. Identical twins and cloned tissue are MHC matched, and are therefore not subject to T-cell mediated rejection. The first successful organ transplant was performed between identical twins by Dr. Joseph Murray at the Peter Bent Brigham Hospital in Boston. This transplant was successful because no T-cell mediated responses were generated to the transplanted organ. Dr. Murray later received a Nobel prize for his work.
The diagnosis of acute rejection relies on the clinical data including patient signs and symptoms, laboratory testing and ultimately a liver biopsy. The biopsy is intrepretated by a pathologist who notes changes in the tissue that suggest rejection. Histologically acute rejection is charaterized by three main features. First, a predominately T-cell rich lymphocytic infiltrate is often present and may be accompanied by a heterogeneous infiltrate including eosinophils, scattered plasma cells and neutrophils. Of note, an abundance of eosinophils within the mixed infiltrate is a helpful feature of acute rejection. Secondly, evidence of injury to the bile ducts is often seen, manifested by the presence of intrepithelial lymphocytes and loss of epithelial cell polarity. Lastly, injury to the vessels may be seen as endothelialitis. Typically this involves portal vein branches, but may include central veins and sinusoids. For a pathologist who evaluates biopsies for liver disease following transplantation, it is important to be aware of the disorders that commonly occur in this setting and their histologic differences. These include autoimmune hepatitis, which will often have a large number of plasma cells; post-transplant lymphoproliferative disorder with it's characteristic monotonous infiltrate and primary biliary cirrhosis which may have focal injury to bile ducts unlike the more monotonous process seen in acute rejection.
Acute Transplant Rejection: A Case Example
Clinical Summary
A 34-year-old white male with end-stage chronic glomerulonephritis had been receiving hemodialysis three times per week for 4 months when he was admitted to the hospital for a living related-donor transplantation from his mother. Other than the kidney disease, the patient was in good health. The transplant was performed successfully with no complications. However, eight days later, transplant rejection necessitated returning the patient to the operating room for a nephrectomy of the transplanted kidney. After the nephrectomy, the patient did quite well, was returned to hemodialysis, and was discharged home in good condition.
Pathological Findings
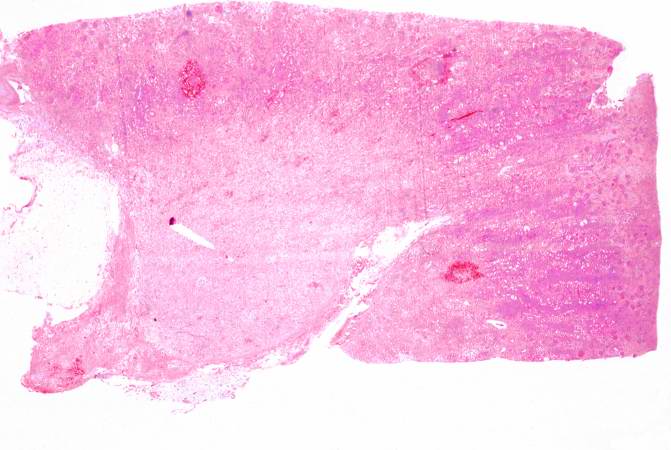
The kidney weighed 240 grams and was edematous. The capsule stripped with ease to reveal a pale tan-brown cortex which was irregularly red-mottled. Upon sectioning, the cortical band was ill-defined, and the corticomedullary junction was not well-demarcated. The renal papillae were edematous and the renal pelvis displayed generalized petechial hemorrhages which extended through the 7-cm segment of ureter to a diffusely hemorrhagic terminal portion.
-
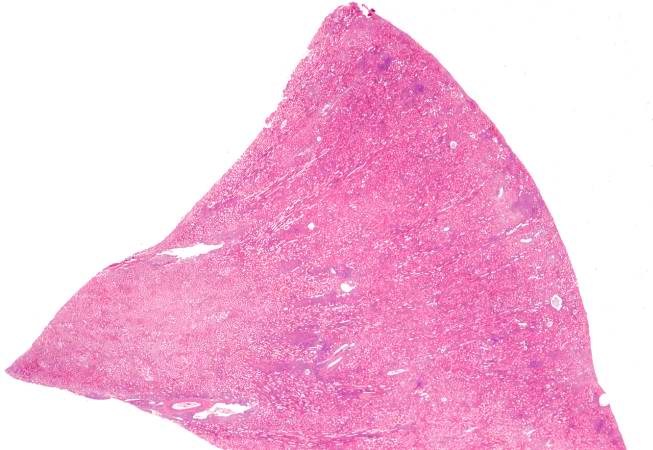
This is a gross photograph of a kidney with acute rejection from an autopsy case. Note that the kidney is swollen (edema and inflammation) and there are areas of hemorrhage throughout the kidney.
-
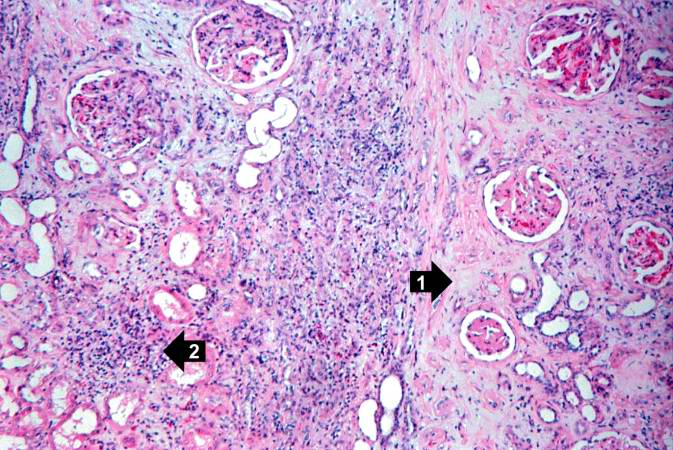
This is a low-power photomicrograph of the kidney that was removed from this patient. Even at this low power you can appreciate the focal accumulations of cells within this section and the diffuse cellular infiltrate (blue dots) throughout the kidney parenchyma.
-
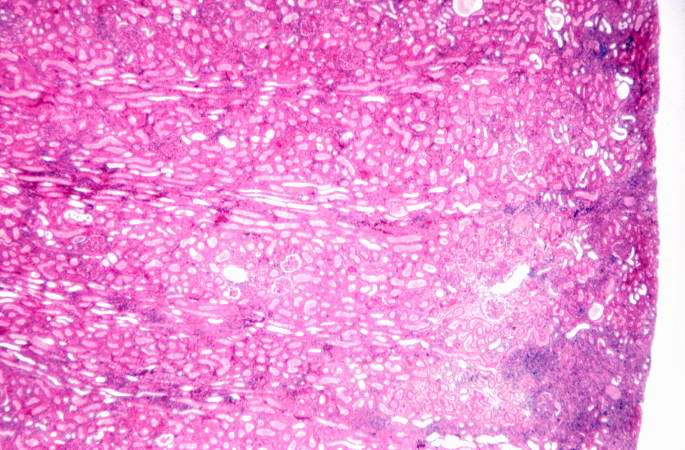
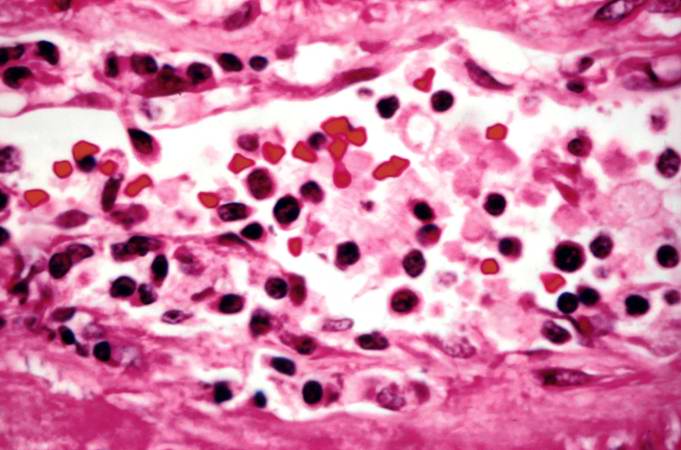
This is a higher-power photomicrograph demonstrating the cellular infiltrates within this kidney section.
-
This is a higher-power photomicrograph demonstrating the cellular infiltrates within this kidney section. Note that in addition to the diffuse cellularity, the focal accumulations of cells appear to be focused around blood vessels.
-
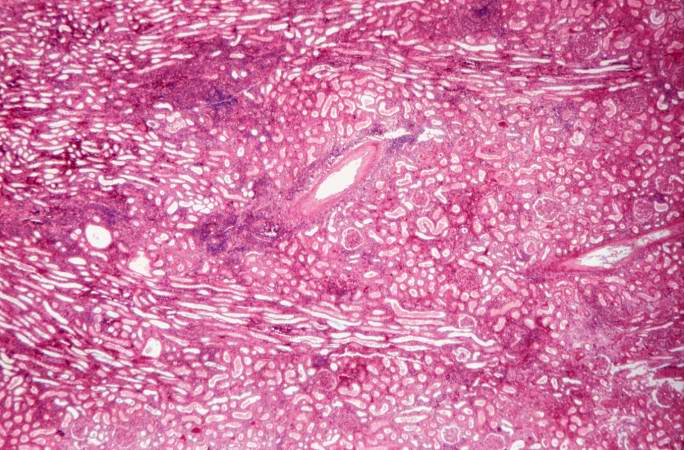
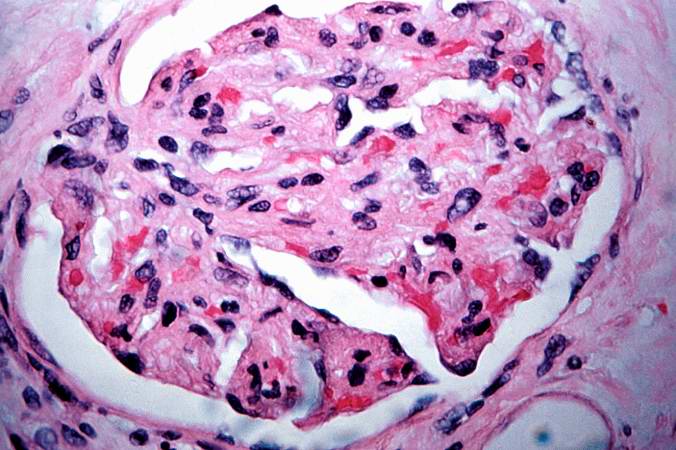
This is a higher-power photomicrograph demonstrating the cellular infiltrate within the interstitium and around the small blood vessel in the center of the image.
-
This is a higher-power photomicrograph demonstrating the cellular infiltrate within the interstitium. There is some degeneration (coagulative necrosis) of tubules and glomeruli.
-
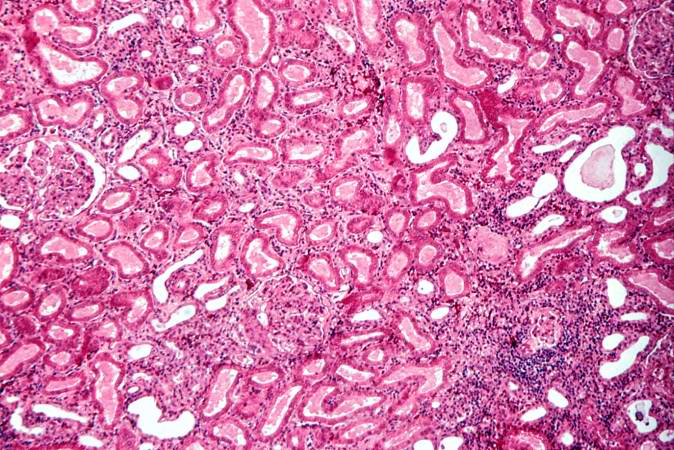
This high-power photomicrograph demonstrates the cellular infiltrate within the interstitium and in the wall of the blood vessel on the left.
-
This high-power photomicrograph demonstrates the cellular infiltrate within the interstitium (1) and in the wall of the blood vessel (2).
-
This is a high-power photomicrograph of cells infiltrating the wall of the blood vessel.
-
This high-power photomicrograph demonstrates the cellular infiltrate within the interstitium and cells within the renal tubules.
Chronic rejection
Chronic rejection was a term used to describe all long term loss of function in organ transplants associated with fibrosis of the internal blood vessels of the transplant, but this is now termed chronic [[allograft] vasculopathy and the term chronic rejection is reserved for those cases where the process is shown to be due to a chronic alloreactive immune response. It can be caused by a member of the Minor Histocompatibility Complex such as the H-Y gene of the male Y chromosome. This usually leads to need for a new organ after a decade or so.
Chronic Transplant Rejection: A Case Example
Clinical Summary
A 39-year-old male had malignant hypertension with malignant nephrosclerosis, progressing to chronic renal failure.
He underwent a bilateral nephrectomy for control of his hypertension and received a cadaveric renal transplant. He did well, although he developed diabetes mellitus and had persistent, but less severe controllable hypertension.
Two years following transplantation he was admitted to the hospital for control of his hypertension and evaluation of his chronic rejection. Initial blood pressure while in the hospital was in the range of 160/110 to 160/100 mm Hg. He was placed on a more intensive hypertension regimen, and he gradually became normotensive. He received one hemodialysis treatment prior to discharge.
At the time of discharge, his blood pressure was 100 to 110 over 60 to 70 and he was doing well on dialysis. His BUN was 113 mg/dL and creatinine 5.2 mg/dL, and he had a hematocrit (PCV) of 27%. The patient was again admitted one month later for evaluation of azotemia and for control of his hypertension. It was felt that his chronic rejection was end-stage and that he would have to be dialyzed periodically. He was put on a renal failure diet, and over the period of his hospitalization, his BUN and creatinine finally stabilized at high levels. He tolerated dialysis well, and a transplant nephrectomy was done at 2 1/2 years post transplant. At the time of discharge, the patient's BUN was 78 mg/dL, creatinine 3.6 mg/dL, WBC 5000 cells/cmm, and the PCV was 26%.
Pathological Findings
The kidney weighed 215 grams and was covered by a thick capsule, which was partially adherent to the cortex, but could be stripped from the kidney with slight difficulty. The calyces and pelvis of the kidney appeared normal. The vessels were not prominent. The renal arteries and vein appeared normal.
-
This is a low-power photomicrograph of the kidney from this case of chronic transplant rejection. Note the focal areas of hemorrhage and inflammatory cell infiltrate in this section.
-
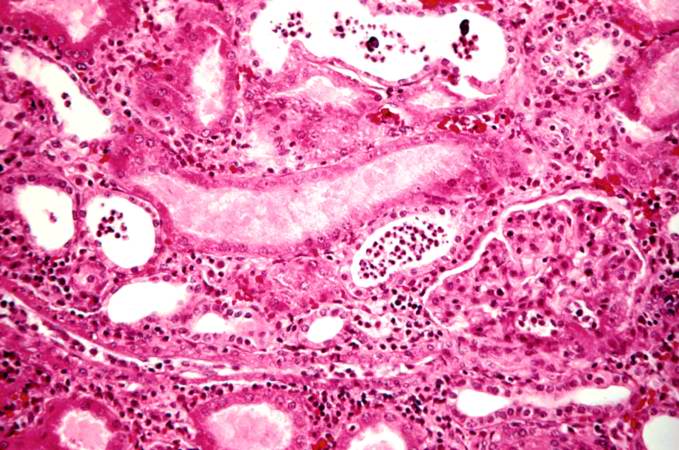
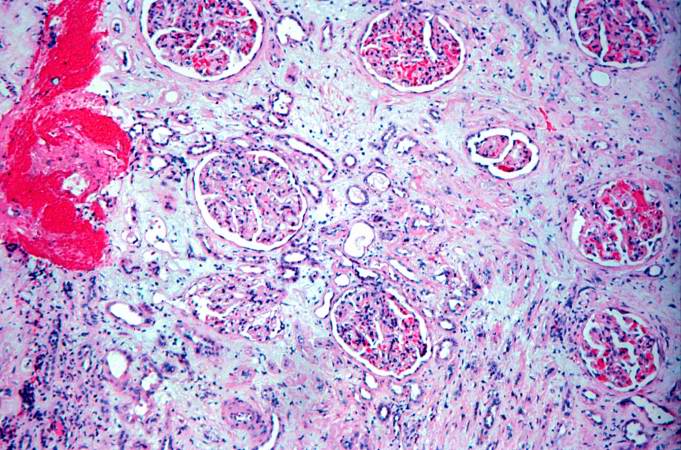
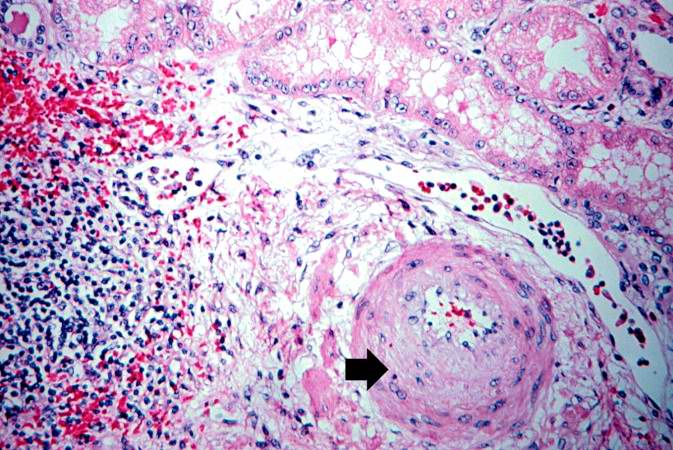
This is a higher-power photomicrograph of kidney containing a section of blood vessel that demonstrates a marked neointimal proliferative response (1). In this case the lumen of the artery is obliterated. Also note the cellular infiltrate in the interstitium of the kidney (2) and the paucity of tubules.
-
This is a photomicrograph of kidney with a focal area of hemorrhage around a small blood vessel (left) and congestion of the glomeruli. Note that there is a marked loss of renal tubules throughout this section with replacement by fibrous connective tissue. Also note the cellularity of the glomeruli.
-
This is another area of renal cortex similar to the previous image. Note the fibrosis (1) and loss of renal tubules throughout this section. Also note the focus of inflammatory cells (2) indicating that despite the chromic nature of this lesion, there is still ongoing active rejection and renal damage.
-
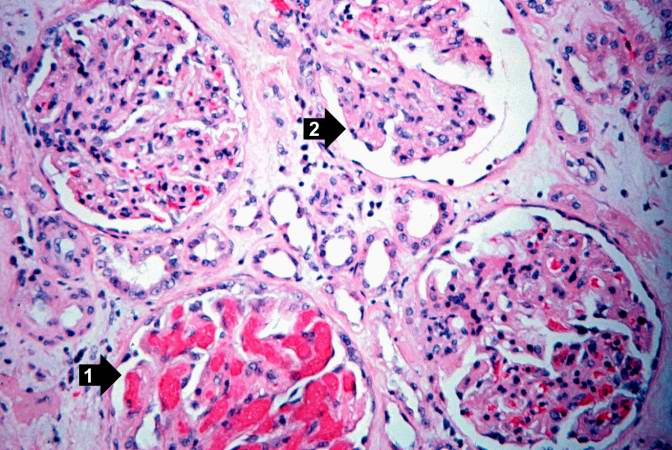
This high-power photomicrograph of glomeruli from this kidney demonstrates congestion (1), increased cellularity of glomeruli with mesangial expansion, and a glomerulus that is almost completely obliterated or sclerosed (2).
-
This is a photomicrograph of rejected kidney with a focus of cellular infiltrate (left) and a small artery with neointimal proliferation and stenosis (arrow).
-
This is a photomicrograph of a glomerulus with a mild cellular infiltrate (left) and a small damaged glomerulus (right). There is extensive interstitial fibrosis (1), loss of renal tubules, and the remaining tubules contain protein (2) indicating severe damage.
-
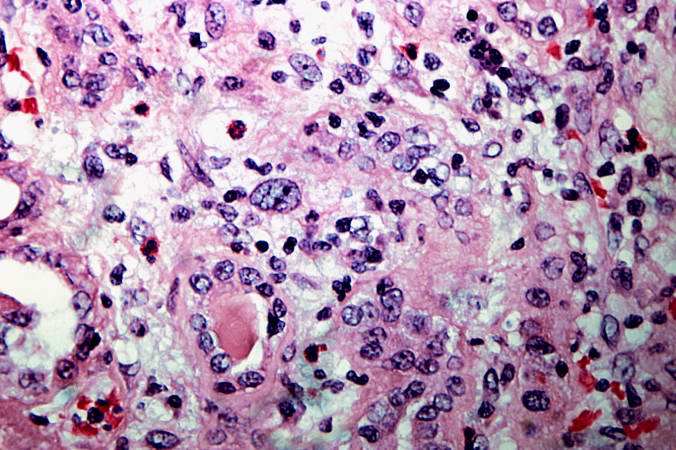
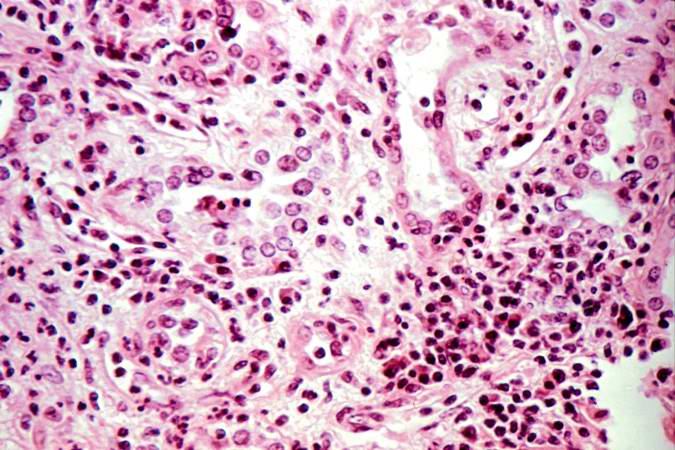
This is a high-power photomicrograph of renal cortex with cellular infiltrate and few remaining renal tubules. The cellular infiltrate comprises macrophages, activated (large) lymphocytes and a few neutrophils and plasma cells.
-
This is a high-power photomicrograph of a damaged glomerulus. Note the loss of normal capillary structure, the mesangial expansion and the infiltration of large mononuclear cells.
-
This is a high-power photomicrograph of a kidney from another case of chronic transplant rejection. In this case there is extensive damage to the kidney due to the chronic rejection (loss of tubules and glomerular lesions). In addition, this kidney was removed during an episode of acute rejection. The marked cellular infiltrate indicates acute rejection in a case of chronic transplant rejection.
-
This is a higher-power photomicrograph of kidney from the previous image demonstrating the cellular infiltrate which is comprised of lymphocytes, macrophages, plasma cells and a few neutrophils.
-
Photomicrograph from another region of previous image. Note the cellular infiltrate around a small blood vessel (right) and neutrophils within renal tubules (arrow).
Rejection mechanisms
Rejection is an adaptive immune response and is mediated through both T cell mediated and humoral immune (antibodies) mechanisms. The number of mismatched alleles determines the speed and magnitude of the rejection response. Different grafts usually have a proclivity to a certain mechanism of rejection.
| Organ/tissue | Mechanism |
|---|---|
| Blood | Antibodies (isohaemagglutinins) |
| Kidney | Antibodies, CMI |
| Heart | Antibodies, CMI |
| Skin | CMI |
| Bonemarrow | CMI |
| Cornea | Usually accepted unless vascularised, CMI |
Causes
Drug Side Effect
Prevention of rejection
Rejection is prevented with a combination of drugs including:
- Calcineurin inhibitors
- mTOR inhibitors
- Anti-proliferatives
- Corticosteroids
- Antibodies
- Monoclonal anti-IL-2Rα receptor antibodies
- Polyclonal anti-T-cell antibodies
- Anti-thymocyte globulin (ATG)
- Anti-lymphocyte globulin (ALG)
Generally a triple therapy regimen of a calcineurin inhibitor, an anti-proliferative, and a corticosteroid is used, although local protocols vary. Antibody inductions can be added to this, especially for high-risk patients and in the United States. mTOR inhibitors can be used to provide calcineurin-inhibitor or steroid-free regimes in selected patients.
A bone marrow transplant allows the chimeric body's immune system to adapt and accept a new organ. This requires that the bone marrow, which produces the immune cells, be from the same person as the organ donation (or an identical twin or a clone). Bone marrow is not attacked by the body's immune system, and is the only known type of transplant that has this quality. However, there is a risk of graft versus host disease (GVHD) in which the immune cells arising from the bone marrow transplant recognise the host tissues as foreign and attack and destroy them accordingly.
An FDA approved immune function test from Cylex has shown effectiveness in minimizing the risk of infection and rejection in post-transplant patients[1] by enabling doctors to tailor immunosuppressant drug regimens. By keeping a patient's immune function within a certain window, doctors could adjust drug levels to prevent organ rejection while avoiding infection. Such information could help physicians reduce the use of immunosuppressive drugs, lowering drug therapy expenses while reducing the morbidity associated with liver biopsies, improve the daily life of transplant patients, and could prolong the life of the transplanted organ.
Treatment of rejection
Acute rejection is normally treated initially with a short course of high-dose methylprednisolone, which is usually sufficient to treat successfully. If this is not enough, the course can be repeated or ATG can be given. Acute rejection refractory to these treatments may require plasma exchanges to remove antibodies to the transplant.
The monoclonal anti-T cell antibody OKT3 was formerly used in the prevention of rejection, and is occasionally used in treatment of severe acute rejection, but has fallen out of common use due to the severe cytokine release syndrome and late post-transplant lymphoproliferative disorder, which are both commonly associated with use of OKT3; in the United Kingdom it is available on a named-patient use basis only.
Acute rejection usually begins after the first week of transplantation, and most likely occurs to some degree in all transplants (except between identical twins). It is caused by mismatched HLA antigens that are present on all cells. HLA antigens are polymorphic therefore the chance of a perfect match is extremely rare. The reason that acute rejection occurs a week after transplantation is because the T-cells involved in rejection must differentiate and the antibodies in response to the allograft must be produced before rejection is initiated. These T-cells cause the graft cells to lyse or produce cytokines that recruit other inflammatory cells, eventually causing necrosis of allograft tissue. Endothelial cells in vascularized grafts such as kidneys are some of the earliest victims of acute rejection. Damage to the endothelial lining is an early predictor of irreversible acute graft failure. The risk of acute rejection is highest in the first 3 months after transplantation, and is lowered by immunosuppressive agents in maintenance therapy. The onset of acute rejection is combatted by episodic treatment.
Chronic rejection is irreversible and cannot be treated effectively. The only definitive treatment is re-transplantation, if necessary. This would typically be ten years after a transplant, and this may entail returning to a transplant queue.
References
External links
Template:Organ transplantation