Raloxifene detailed information
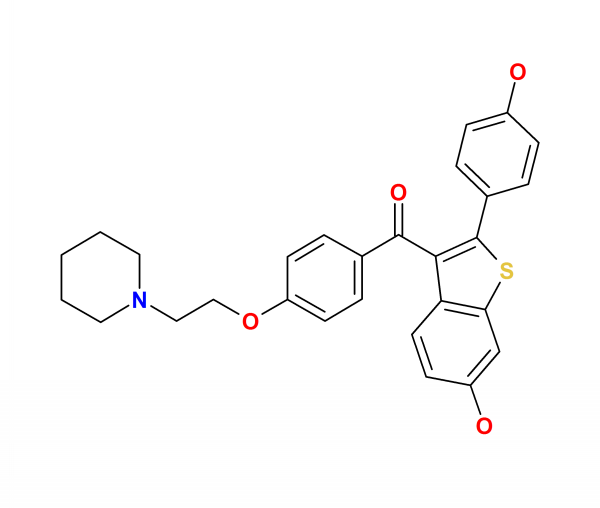 | |
| Clinical data | |
|---|---|
| [[Regulation of therapeutic goods |Template:Engvar data]] | |
| Pregnancy category | |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 2% |
| Protein binding | 95% |
| Metabolism | Hepatic glucuronidation CYP system not involved |
| Elimination half-life | 27.7 hours |
| Excretion | Fecal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C28H27NO4S |
| Molar mass | 473.584 g/mol |
Raloxifene is an oral selective estrogen receptor modulator which is used in the prevention of osteoporosis in postmenopausal women. It was announced on April 17, 2006, that raloxifene is as effective as tamoxifen in reducing the incidence of breast cancer in certain high risk groups of females, [1] though with a reduced risk of thromboembolic events and cataracts in patients taking raloxifene versus those taking tamoxifen.[1] On September 14, 2007, the U.S. Food and Drug Administration announced approval of raloxifene for reducing the risk of invasive breast cancer in postmenopausal women with osteoporosis and in postmenopausal women at high risk for invasive breast cancer.[2]
There has been criticism in the mainstream oncology press of the way that information about the drug was released.[3] There has been some confusion in the lay media about the meaning of the trial results. There is no specific clinical evidence for the use of raloxifene in the adjuvant treatment of breast cancer over established drugs such as tamoxifen or anastrozole.[citation needed]
Raloxifene is produced by Eli Lilly Pharmaceuticals and is sold under the brand name Evista®.
Description
Raloxifene hydrochloride (HCl) has the empirical formula C28H27NO4S•HCl, which corresponds to a molecular weight of 510.05 g/mol. Raloxifene HCl is an off-white to pale-yellow solid that is slightly soluble in water.
SERMs mimic estrogen in some tissues and have anti-estrogen activity in others. Other SERMs, such as Pfizer's lasofoxifene and Wyeth's bazedoxifene are in the late
Indication
Raloxifene is indicated for the treatment and prevention of osteoporosis in postmenopausal women, for reduction in risk of invasive breast cancer in postmenopausal women with osteoporosis, and for reduction in risk of invasive breast cancer in postmenopausal women at high risk for invasive breast cancer.
For either osteoporosis treatment or prevention, supplemental calcium and/or vitamin D should be added to the diet if daily intake is inadequate.
Contraindications and precautions
Raloxifene is contraindicated in lactating women or women who are or may become pregnant, in women with active or past history of venous thromboembolic events, including deep vein thrombosis, pulmonary embolism, and retinal vein thrombosis and in women known to be hypersensitive to raloxifene.
Adverse reactions
Common adverse events considered to be drug-related were hot flashes and leg cramps.
Raloxifene may infrequently cause serious blood clots to form in the legs, lungs, or eyes. Other reactions experienced include leg swelling/pain, trouble breathing, chest pain, vision changes.
As cancer drug
Raloxifene reduces the risk of hormone-positive breast cancer and vertebral fractures "without a shadow of a doubt," but its effects on cardiovascular disease remain less certain, according to the results of the "Raloxifene for Use of the Heart" (RUTH) study published in the July 13, 2006 issue of the New England Journal of Medicine by Dr. Elizabeth Barrett-Connor (University of California at San Diego) and colleagues.[4]
In the trial, in women with coronary heart disease (CHD) or multiple risk factors for CHD, raloxifene had no significant effect on the primary end point, coronary events, but it did significantly increase the risk of venous thromboembolism (VTE). And although the drug had no effect on stroke, there was a seemingly paradoxical significant increase in death from stroke.[5]
On September 14, 2007, Steven Galson, director of the United States Food and Drug Administration's Center for Drug Evaluation and Research announced authorization of the sale of raloxifene to prevent invasive breast cancer in post-menopausal women.[6]
References
- ↑ Vogel, Victor. "Effects of Tamoxifen vs. Raloxifene on the Risk of Developing Invasive Breast Cancer and Other Disease Outcomes". The Journal of the American Medical Association. 295 (23): 2727–2741. Unknown parameter
|coauthors=ignored (help) - ↑ "FDA Approves New Uses for Evista" (Press release). U.S. Food and Drug Administration. 2007-09-14. Retrieved 2007-09-15.
- ↑ "A STARring role for raloxifene?". Lancet Oncol. 7 (6): 443. 2006. PMID 16750489.
- ↑ Lisa Nainggolan (July 12, 2006). "A balancing act: The pro and cons of raloxefene".
- ↑ Barrett-Connor E, Mosca L, Collins P; et al. (2006). "Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women". New England Journal of Medicine. 355: 125–137.
- ↑ AFP.google.com, US approves Lilly's Evista for breast cancer prevention
- Heringa M (2003). "Review on raloxifene: profile of a selective estrogen receptor modulator". Int J Clin Pharmacol Ther. 41 (8): 331–45. PMID 12940590.
- Barrett-Connor E. "Raloxifene: risks and benefits". Ann N Y Acad Sci. 949: 295–303. PMID 11795366.
External links
- STAR: a head-to-head comparison of tamoxifen and raloxifene as breast-cancer preventatives
- Full Prescribing Information
- Position paper of the National Women's Health Network
- Pages with script errors
- Pages with citations using unsupported parameters
- CS1 maint: Explicit use of et al.
- CS1 maint: Multiple names: authors list
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drug has EMA link
- Articles containing unverified chemical infoboxes
- All articles with unsourced statements
- Articles with unsourced statements from September 2007
- Articles with invalid date parameter in template
- Benzothiophenes
- Lilly
- Piperidines
- Selective estrogen receptor modulators
- Endocrinology