Polidocanol
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Polidocanol is a sclerosing agent that is FDA approved for the treatment of uncomplicated spider veins (varicose veins ≤1 mm in diameter) and uncomplicated reticular veins (varicose veins 1 to 3 mm in diameter) in the lower extremity. Common adverse reactions include mild local reactions at the site of injection.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Asclera® (polidocanol) is indicated to sclerose uncomplicated spider veins (varicose veins ≤1 mm in diameter) and uncomplicated reticular veins (varicose veins 1 to 3 mm in diameter) in the lower extremity. Asclera has not been studied in varicose veins more than 3 mm in diameter.
Dosage
- For intravenous use only. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if particulate matter is seen or if the contents of the vial are discolored or if the vial is damaged in any way.
- For spider veins (varicose veins ≤1 mm in diameter), use Asclera 0.5%. For reticular veins (varicose veins 1 to 3 mm in diameter), use Asclera 1%. Use 0.1 to 0.3 mL per injection and no more than 10 mL per session.
- Use a syringe (glass or plastic) with a fine needle (typically, 26- or 30-gauge). Insert the needle tangentially into the vein and inject the solution slowly while the needle is still in the vein. Apply only gentle pressure during injection to prevent vein rupture. After the needle has been removed and the injection site has been covered, apply compression in the form of a stocking or bandage. After the treatment session, encourage the patient to walk for 15 to 20 minutes. Keep the patient under observation to detect any anaphylactic or allergic reaction.
- Maintain compression for 2 to 3 days after treatment of spider veins and for 5 to 7 days for reticular veins. For extensive varicosities, longer compression treatment with compression bandages or a gradient compression stocking of a higher compression class is recommended. Post-treatment compression is necessary to reduce the risk of deep vein thrombosis.
- Repeat treatments may be necessary if the extent of the varicose veins requires more than 10 mL. These treatments should be separated by 1 to 2 weeks.
- Small intravaricose blood clots (thrombi) that develop may be removed by stab incision and thrombus expression (microthrombectomy).
DOSAGE FORMS AND STRENGTHS
- Asclera is available as a 0.5% and 1% solution in 2 mL glass ampules.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Polidocanol in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Polidocanol in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Polidocanol in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Polidocanol in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Polidocanol in pediatric patients.
Contraindications
- Asclera is contraindicated for patients with known allergy (anaphylaxis) to polidocanol and patients with acute thromboembolic diseases.
Warnings
Anaphylaxis
- Severe allergic reactions have been reported following polidocanol use, including anaphylactic reactions, some of them fatal. Severe reactions are more frequent with use of larger volumes (> 3 mL). The dose of polidocanol should therefore be minimized. Be prepared to treat anaphylaxis appropriately.
- Severe adverse local effects, including tissue necrosis, may occur following extravasation; therefore, care should be taken in intravenous needle placement and the smallest effective volume at each injection site should be used.
- After the injection session is completed, apply compression with a stocking or bandage, and have the patient walk for 15-20 minutes. Keep the patient under supervision during this period to treat any anaphylactic or allergic reaction.
Accidental Intra-arterial Injection
- Intra-arterial injection can cause severe necrosis, ischemia or gangrene. If this occurs consult a vascular surgeon immediately.
Inadvertent Perivascular Injection
- Inadvertent perivascular injection of Asclera can cause pain. If pain is severe, a local anesthetic (without adrenaline) may be injected.
Adverse Reactions
Clinical Trials Experience
Clinical Study Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- In 5 controlled randomized clinical trials, Asclera has been administered to 401 patients with small or very small varicose veins (reticular and spider veins) and compared with another sclerosing agent and with placebo. Patients were 18 to 70 years old. The patient population was predominately female and consisted of Caucasian and Asian patients.
- Table 1 shows adverse events more common with Asclera or sodium tetradecyl sulfate (STS) 1% than with placebo by at least 3% in the placebo- controlled EASI study. All of these were injection site reactions and most were mild.
- Ultrasound examinations at one week (±3 days) and 12 weeks (±2 weeks) after treatment did not reveal deep vein thrombosis in any treatment group.
Postmarketing Experience
Post-marketing Safety Experience
- The following adverse reactions have been reported during use of polidocanol in world-wide experience; in some of these cases these adverse events have been serious or troublesome. Because these reactions are reported voluntarily from a population of uncertain size and without a control group, it is not possible to estimate their frequency reliably or to establish a causal relationship to drug exposure.
Immune system disorders
- Anaphylactic shock, angioedema, urticaria generalized, asthma
Nervous system disorders
- Cerebrovascular accident, migraine, paresthesia (local), loss of consciousness, confusional state, dizziness
Cardiac disorders
Vascular disorders
Respiratory, thoracic and mediastinal disorders
Skin and subcutaneous tissue disorders
- Skin hyperpigmentation, allergic dermatitis, hypertrichosis (in the area of sclerotherapy)
General disorders and injection site conditions
Injury, poisoning and procedural complications
Drug Interactions
- No drug-drug interactions have been studied with Asclera.
Use in Specific Populations
Pregnancy
- Polidocanol has been shown to have an embryocidal effect in rabbits when given in doses approximately equal (on the basis of body surface area) to the human dose. This effect may have been secondary to maternal toxicity. There are no adequate and well-controlled studies in pregnant women. Asclera should not be used during pregnancy.
Animal Studies
- Developmental reproductive toxicity testing was performed in rats and rabbits with intravenous administration. Polidocanol induced maternal and fetal toxicity in rabbits, including reduced mean fetal weight and reduced fetal survival, when administered during gestation days 6-20 at doses of 4 and 10 mg/kg, but it did not cause skeletal or visceral abnormalities. No adverse maternal or fetal effects were observed in rabbits at a dose of 2 mg/kg. No evidence of teratogenicity or fetal toxicity was observed in rats dosed during gestation days 6-17 with doses up to 10 mg/kg. Polidocanol did not affect the ability of rats to deliver and rear pups when administered intermittently by intravenous injection from gestation day 17 to post-partum day 21 at doses up to 10 mg/kg.
Human Studies
- There are no adequate and well-controlled studies on the use of Asclera in pregnant women.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Polidocanol in women who are pregnant.
Labor and Delivery
- The effects of Asclera on labor and delivery in pregnant women are unknown.
Nursing Mothers
- It is not known whether polidocanol is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants, avoid administering to a nursing woman.
Pediatric Use
- The safety and effectiveness of Asclera in pediatric patients have not been established.
Geriatic Use
- Clinical studies of Asclera did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
Gender
There is no FDA guidance on the use of Polidocanol with respect to specific gender populations.
Race
There is no FDA guidance on the use of Polidocanol with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Polidocanol in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Polidocanol in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Polidocanol in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Polidocanol in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
Monitoring
There is limited information regarding Monitoring of Polidocanol in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Polidocanol in the drug label.
Overdosage
- Overdose may result in a higher incidence of localized reactions such as necrosis.
Pharmacology
Mechanism of Action
- The active ingredient of Asclera is polidocanol.
- Polidocanol is a sclerosing agent that locally damages the endothelium of blood vessels. When injected intravenously, polidocanol induces endothelial damage. Platelets then aggregate at the site of damage and attach to the venous wall. Eventually, a dense network of platelets, cellular debris, and fibrin occludes the vessel. Finally, the occluded vein is replaced with connective fibrous tissue.
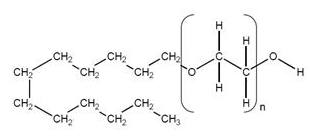
Structure
- Asclera is a sterile, nonpyrogenic, and colorless to faintly greenish-yellow solution of polidocanol for intravenous use as a sclerosing agent.
- The active ingredient, polidocanol is a non-ionic detergent, consisting of two components, a polar hydrophilic (dodecyl alcohol) and an apolar hydrophobic (polyethylene oxide) chain. Polidocanol has the following structural formula:
- C12H25(OCH2CH2)nOH Polyethylene glycol monododecyl ether Mean extent of polymerization (n) : Approximately 9 Mean molecular weight : Approximately 600
- Each mL contains 5 mg (0.5%) or 10 mg (1.0%) polidocanol in water for injection with 5% (v/v) ethanol at pH 6.5-8.0; disodium hydrogen phosphate dihydrate, potassium dihydrogen phosphate are added for pH adjustment.
Pharmacodynamics
- Polidocanol has a concentration- and volume-dependent damaging effect on the endothelium of blood vessels.
Pharmacokinetics
- During the major effectiveness study (EASI-trial), scheduled blood samples were taken from a sub-group of 22 patients to measure plasma levels of polidocanol after Asclera treatment of spider and reticular veins. Low systemic blood levels of polidocanol were seen in some patients.
- The mean t1/2 of polidocanol in 4 patients with evaluable data receiving 4.5 -18.0 mg was 1.5 h.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Long-term studies to evaluate carcinogenic potential have not been conducted with polidocanol. Polidocanol was negative in bacterial reverse mutation assays in Salmonella and E. coli, and in a micronucleus assay conducted in mice. Polidocanol induced numerical chromosomal aberrations in cultured newborn Chinese hamster lung fibroblasts in the absence of metabolic activation.
- Polidocanol did not affect reproductive performance (fertility) of rats when administered intermittently at dosages up to 10 mg/kg (approximately equal to the maximum human dose on the basis of body surface area).
Clinical Studies
- Asclera was evaluated in a multicenter, randomized, double-blind, placebo- and comparator-controlled trial (EASI-study) in patients with spider or reticular varicose veins. A total of 338 patients were treated with Asclera [0.5% for spider veins (n=94), 1% for reticular veins (n=86)], sodium tetradecyl sulfate (STS) 1% (n=105), or placebo (0.9% isotonic saline solution) (n=53) for either spider or reticular veins. Patients were predominately female, ranging in age from 19 to 70 years. All of them received an intravenous injection in the first treatment session; repeat injections were given three and six weeks later if the previous injection was evaluated as unsuccessful (defined as 1, 2 or 3 on a 5-point scale, see below). Patients returned at 12 and 26 weeks after the last injection for final assessments.
- The primary effectiveness endpoint was improvement of veins judged by a blinded panel. Digital images of the selected treatment area were taken prior to injection, compared with those taken at 12 weeks post-treatment, and rated on a 5-point scale (1 = worse than before, 2 = same as before, 3 = moderate improvement, 4 = good improvement, 5 = complete treatment success); results are shown in Table 2.
- The secondary efficacy criterion was the rate of treatment success, pre-defined as a score of 4 or 5 with patients scoring 1, 2, or 3 considered treatment failures; results are shown in Table 3.
- At 12 and 26 weeks, patients' judgement of the results was assessed by showing them the digital images of their treatment area taken at baseline and asking them to rate their satisfaction with their treatment using a verbal rating scale (1 = very unsatisfied; 2 = somewhat unsatisfied; 3 = slightly satisfied; 4 = satisfied and 5 = very satisfied); results are shown in Table 4.
How Supplied
- Asclera is supplied in single-use, preservative free ampules in the following packages:
- NDC 46783-121-52 Five 0.5% ampules (2 mL)
- NDC 46783-221-52 Five 1.0% ampules (2 mL)
- Each ampule is intended for immediate use in a single patient. Each unopened ampule is stable up to three years.
Storage
- Store at 15-30°C; (59-86°F).
Images
Drug Images
{{#ask: Page Name::Polidocanol |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
PRINCIPAL DISPLAY PANEL - 20 MG AMPULE CARTON
NDC 46783-221-52
ASCLERA® (polidocanol) Injection
20 mg per 2 mL (10 mg per mL)
1%
For Intravenous Use Only Rx Only Single use: Discard unused portion Contains: 5 ampules each containing 20 mg per 2 mL
PRINCIPAL DISPLAY PANEL - 10 MG AMPULE CARTON
NDC 46783-121-52
ASCLERA® (polidocanol) Injection
10 mg per 2 mL (5 mg per mL)
0.5%
For Intravenous Use Only Rx Only Single use: Discard unused portion Contains: 5 ampules each containing 10 mg per 2 mL
Ingredients and Appearance
{{#ask: Label Page::Polidocanol |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise the patient to wear compression stockings or support hose on the treated legs continuously for 2 to 3 days and for 2 to 3 weeks during the daytime. Compression stockings or support hose should be thigh or knee high depending upon the area treated in order to provide adequate coverage.
- Advise the patient to walk for 15 to 20 minutes immediately after the procedure and daily for the next few days.
- For two to three days following treatment, advise the patient to avoid heavy exercise, sunbathing, long plane flights, and hot baths or sauna.
Precautions with Alcohol
- Alcohol-Polidocanol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ASCLERA®[1]
Look-Alike Drug Names
There is limited information regarding Polidocanol Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.