Dexchlorpheniramine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Dexchlorpheniramine is a antihistamine that is FDA approved for the treatment of Perennial and seasonal allergic rhinitis, Vasomotor rhinitis, Allergic conjunctivitis, Mild, uncomplicated allergic skin manifestations of urticaria and angioedema, Amelioration of allergic reactions to blood or plasma, Dermographism.. Common adverse reactions include Diarrhea, Epigastric discomfort, Nausea, Vomiting, Xerostomia, Somnolence ,Nasal mucosa dry.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Perennial and seasonal allergic rhinitis
- Vasomotor rhinitis
- Allergic conjunctivitis due to inhalant allergens and foods
- Mild, uncomplicated allergic skin manifestations of urticaria and angioedema
- Amelioration of allergic reactions to blood or plasma
- Dermographism
- As therapy for anaphylactic reactions adjunctive to epinephrine and other standard measures after the acute manifestations have been controlled.
Dosage
- DOSAGE SHOULD BE INDIVIDUALIZED ACCORDING TO THE NEEDS AND THE RESPONSE OF THE PATIENT.
Recommended Dosage
- Adults and Children 12 years of age and older: 2 mg (1 teaspoonful)
- Doses are generally given every 4 to 6 hours.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Dexchlorpheniramine in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Dexchlorpheniramine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Dosage
- Children 6 to 11 years: 1 mg (1/2 teaspoonful)
- Children 2 to 5 years: 0.5 mg (1/4 teaspoonful)
- Doses are generally given every 4 to 6 hours.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Dexchlorpheniramine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Dexchlorpheniramine in pediatric patients.
Contraindications
Use in Newborn or Premature Infants
- This drug should not be used in newborn or premature infants.
Use in Nursing Mothers
- Because of the higher risk of antihistamines for infants generally and for newborns and prematures in particular, antihistamine therapy is contraindicated in nursing mothers.
Use in Lower Respiratory Disease
- Antihistamines should NOT be used to treat lower respiratory tract symptoms including asthma.
Antihistamines are also contraindicated in the following conditions
- Hypersensitivity to dexchlorpheniramine maleate or other antihistamines of similar chemical structure
- Monoamine oxidase inhibitor therapy
Warnings
- Antihistamines should be used with considerable caution in patients with:
Use with CNS Depressants
- Dexchlorpheniramine Maleate, USP has additive effects with alcohol and other CNS depressants (hypnotics, sedatives, tranquilizers, etc.).
Use in Activities Requiring Mental Alertness
- Patients should be warned about engaging in activities requiring mental alertness such as driving a car or operating appliances, machinery, etc.
Precautions
- Dexchlorpheniramine Maleate, USP has an atropine-like action and, therefore, should be used with caution in patients with:
- History of bronchial asthma
- Increased intraocular pressure
- Hyperthyroidism
- Cardiovascular disease
- Hypertension
Adverse Reactions
Clinical Trials Experience
General
- Urticaria, drug rash, anaphylactic shock, photosensitivity, excessive perspiration, chills, dryness of mouth, nose and the throat.
Hematologic System
Nervous System
- Sedation, sleepiness, dizziness, disturbed coordination, fatigue, confusion, restlessness, excitation, nervousness, tremor, irritability, insomnia, euphoria, paresthesias, blurred vision, diplopia, vertigo, tinnitus, acute labyrinthitis, hysteria, neuritis, convulsions.
G.I. System
G.U. System
Respiratory System
- Thickening of bronchial secretions, tightness of chest and wheezing, nasal stuffiness.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Dexchlorpheniramine in the drug label.
Drug Interactions
- MAO inhibitors prolong and intensify the anticholinergic (drying) effects of antihistamines.
Use in Specific Populations
Pregnancy
- Experience with this drug in pregnant women is inadequate to determine whether there exists a potential for harm to the developing fetus.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Dexchlorpheniramine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Dexchlorpheniramine during labor and delivery.
Nursing Mothers
- Because of the higher risk of antihistamines for infants generally and for newborns and prematures in particular, antihistamine therapy is contraindicated in nursing mothers.
Pediatric Use
- In infants and children, especially, antihistamines in overdosage may cause hallucinations, convulsions, or death.
As in adults, antihistamines may diminish mental alertness in children. In the young child, particularly, they may produce excitation.
Geriatic Use
- Antihistamines are more likely to cause dizziness, sedation, and hypotension in elderly patients.
Gender
There is no FDA guidance on the use of Dexchlorpheniramine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Dexchlorpheniramine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Dexchlorpheniramine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Dexchlorpheniramine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Dexchlorpheniramine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Dexchlorpheniramine in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Dexchlorpheniramine in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Dexchlorpheniramine in the drug label.
Overdosage
- Antihistamine overdosage reactions may vary from central nervous system depression to stimulation. Stimulation is particularly likely in children. Atropine-like signs and symptoms—dry mouth, fixed, dilated pupils, flushing, and gastrointestinal symptoms may also occur.
- If vomiting has not occurred spontaneously the patient should be induced to vomit. This is best done by having the patient drink a glass of water or milk after which the patient should be made to gag. Precautions against aspiration must be taken, especially in infants and children.
- Saline cathartics, such as milk of magnesia, draw water into the bowel by osmosis and therefore, are valuable for their action in rapid dilution of bowel content.
- Stimulants should not be used.
- Vasopressors may be used to treat hypotension.
Pharmacology
| |
Chlorpheniramine
| |
| Systematic (IUPAC) name | |
| (3S)-3-(4-chlorophenyl)-N,N-dimethyl-3-pyridin-3-ylpropan-1-amine | |
| Identifiers | |
| CAS number | |
| ATC code | R06 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 274.788 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | Oral |
Mechanism of Action
- Dexchlorpheniramine maleate is an antihistamine with anticholinergic (drying) and sedative side effects. Antihistamines appear to compete with histamine for cell receptor sites on effector cells.
Structure
- Each 5 mL (teaspoonful) contains:
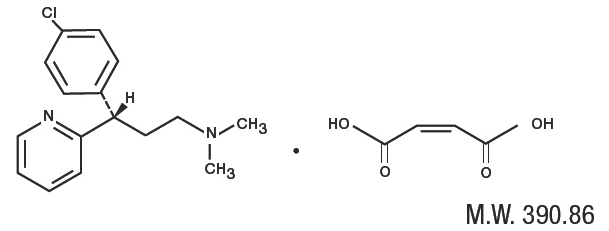
- Dexchlorpheniramine Maleate, USP, an antihistamine agent, is a white, odorless crystalline powder that is freely soluble in water. The molecular formula is C16H19CIN2•C4H4O4, designated chemically as (+)-2-[p-Chloro-α-[2-(dimethylamino)ethyl]benzyl]pyridine maleate (1:1).
Inactive Ingredients
- Citric Acid; Dehydrated Alcohol; FD&C Red No. 40; Glycerin; Liquid Sugar; Menthol; Methylparaben; Natural and Artificial Orange Juice Flavor; Propylene Glycol; Propylparaben and Purified Water. May also contain Sodium Citrate for pH adjustment. The pH range is between 5.0 and 6.5.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Dexchlorpheniramine in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Dexchlorpheniramine in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Dexchlorpheniramine in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Dexchlorpheniramine in the drug label.
How Supplied
- Dexchlorpheniramine Maleate Oral Solution, USP 2 mg/5 mL is supplied as a red-orange colored, orange flavored liquid in the following sizes:
- 16 fl oz (473 mL)
Storage
- Store at (20 – 25 )°C ((68 – 77)°F) [See USP Controlled Room Temperature].
- Dispense in a tight, light-resistant container as defined in the USP, with child-resistant closure.
Images
Drug Images
{{#ask: Page Name::Dexchlorpheniramine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
PRINCIPAL DISPLAY PANEL - 473 ML BOTTLE LABEL
MGP
NDC 60432-539-16
DEXCHLORPHENIRAMINE MALEATE ORAL SOLUTION, USP
2 mg/5 mL
(Contains alcohol not more than 7.0%)
DO NOT USE IF INNER FOIL SEAL PRINTED "SEALED FOR YOUR PROTECTION" IS BROKEN OR MISSING.
BULK CONTAINER — NOT FOR HOUSEHOLD USE
Rx Only
NET: 1 Pint (473 mL)
Ingredients and Appearance
{{#ask: Label Page::Dexchlorpheniramine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Dexchlorpheniramine in the drug label.
Precautions with Alcohol
- Alcohol-Dexchlorpheniramine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Polaramine®[1]
Look-Alike Drug Names
There is limited information regarding Dexchlorpheniramine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.