Ofloxacin (otic)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Adeel Jamil, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Ofloxacin (otic) is a fluroquinolones and anti-bacterial agent that is FDA approved for the treatment of otitis externa, chronic suppurative otitis media and acute otitis media. Common adverse reactions include pruritus, earache, vertigo, headache, dizziness and rash.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Ofloxacin otic solution 0.3% is indicated for the treatment of infections caused by susceptible isolates of the designated microorganisms in the specific conditions listed below:
Otitis Externa
- Otitis Externa in adults and pediatric patients, 6 months and older, due to Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus.
Chronic Suppurative Otitis Media
- Chronic Suppurative Otitis Media in patients 12 years and older with perforated tympanic membranes due to Proteus mirabilis, Pseudomonas aeruginosa, and Staphylococcus aureus.
Acute Otitis Media
- Acute Otitis Media in pediatric patients one year and older with tympanostomy tubes due to Haemophilus influenzae, Moraxella catarrhalis, Pseudomonas aeruginosa, Staphylococcus aureus,and Streptococcus pneumoniae.
Dosing Information
Otitis Externa
- The recommended dosage regimen for the treatment of otitis externa is:
- For pediatric patients (from 6 months to 13 years old): Five drops (0.25 mL, 0.75 mg ofloxacin) instilled into the affected ear once daily for seven days.
- For patients 13 years and older: Ten drops (0.5 mL, 1.5 mg ofloxacin) instilled into the affected ear once daily for seven days.
- The solution should be warmed by holding the bottle in the hand for one or two minutes to avoid dizziness which may result from the instillation of a cold solution. The patient should lie with the affected ear upward, and then the drops should be instilled. This position should be maintained for five minutes to facilitate penetration of the drops into the ear canal. Repeat, if necessary, for the opposite ear.
Acute Otitis Media in Pediatric Patients with Tympanostomy Tubes
- The recommended dosage regimen for the treatment of acute otitis media in pediatric patients (from one to 12 years old) with tympanostomy tubes is:
- Five drops (0.25 mL, 0.75 mg ofloxacin) instilled into the affected ear twice daily for ten days. The solution should be warmed by holding the bottle in the hand for one or two minutes to avoid dizziness that may result from the instillation of a cold solution. The patient should lie with the affected ear upward, and then the drops should be instilled. The tragus should then be pumped 4 times by pushing inward to facilitate penetration of the drops into the middle ear. This position should be maintained for five minutes. Repeat, if necessary, for the opposite ear.
Chronic Suppurative Otitis Media With Perforated Tympanic Membranes
- The recommended dosage regimen for the treatment of chronic suppurative otitis media with perforated tympanic membranes in patients 12 years and older is:
- Ten drops (0.5 mL, 1.5 mg ofloxacin) instilled into the affected ear twice daily for fourteen days. The solution should be warmed by holding the bottle in the hand for one or two minutes to avoid dizziness that may result from the instillation of a cold solution. The patient should lie with the affected ear upward, before instilling the drops. The tragus should then be pumped 4 times by pushing inward to facilitate penetration into the middle ear. This position should be maintained for five minutes. Repeat, if necessary, for the opposite ear.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ofloxacin (otic) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ofloxacin (otic) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Ofloxacin (otic) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ofloxacin (otic) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ofloxacin (otic) in pediatric patients.
Contraindications
- Ofloxacin otic solution 0.3% is contraindicated in patients with a history of hypersensitivity to ofloxacin, to other quinolones, or to any of the components in this medication.
Warnings
- Not for ophthalmic use.
- Not for injection.
- Serious and occasionally fatal hypersensitivity (anaphylactic) reactions, some following the first dose, have been reported in patients receiving systemic quinolones, including ofloxacin. Some reactions were accompanied by cardiovascular collapse, loss of consciousness, angioedema (including laryngeal, pharyngeal or facial edema), airway obstruction, dyspnea, urticaria, and itching. If an allergic reaction to ofloxacin is suspected, stop the drug. Serious acute hypersensitivity reactions may require immediate emergency treatment. Oxygen and airway management, including intubation, should be administered as clinically indicated.
PRECAUTIONS
General
- As with other anti-infective preparations, prolonged use may result in over-growth of nonsusceptible organisms, including fungi. If the infection is not improved after one week, cultures should be obtained to guide further treatment. If otorrhea persists after a full course of therapy, or if two or more episodes of otorrhea occur within six months, further evaluation is recommended to exclude an underlying condition such as cholesteatoma, foreign body, or a tumor.
- The systemic administration of quinolones, including ofloxacin at doses much higher than given or absorbed by the otic route, has led to lesions or erosions of the cartilage in weight-bearing joints and other signs of arthropathy in immature animals of various species.
- Young growing guinea pigs dosed in the middle ear with 0.3% ofloxacin otic solution showed no systemic effects, lesions or erosions of the cartilage in weight-bearing joints, or other signs of arthropathy. No drug-related structural or functional changes of the cochlea and no lesions in the ossicles were noted in the guinea pig following otic administration of 0.3% ofloxacin for one month.
- No signs of local irritation were found when 0.3% ofloxacin was applied topically in the rabbit eye. Ofloxacin was also shown to lack dermal sensitizing potential in the guinea pig maximization study.
Adverse Reactions
Clinical Trials Experience
Subjects with Otitis Externa
- In the phase III clinical trials performed in support of once-daily dosing, 799 subjects with otitis externa and intact tympanic membranes were treated with ofloxacin otic solution. The studies, which served as the basis for approval, were 020 (pediatric, adolescents and adults), 016 (adolescents and adults) and 017 (pediatric). The following treatment-related adverse events occurred in two or more of the subjects.

- An unexpected increased incidence of application site reaction was seen in studies 016/017 and was similar for both ofloxacin and the active control drug (neomycin-polymyxin B sulfate-hydrocortisone). This finding is believed to be the result of specific questioning of the subjects regarding the incidence of application site reactions.
- In once daily dosing studies, there were also single reports of nausea, seborrhea, transient loss of hearing, tinnitus, otitis externa, otitis media, tremor, hypertension and fungal infection.
- In twice daily dosing studies, the following treatment-related adverse events were each reported in a single subject: dermatitis, eczema, erythematous rash, follicular rash, hypoaesthesia, tinnitus, dyspepsia, hot flushes, flushing and otorrhagia.
Subjects with Acute Otitis Media with Tympanostomy Tubes (AOM TT) and Subjects with Chronic Suppurative Otitis Media (CSOM) with Perforated Tympanic Membranes
- In phase III clinical trials which formed the basis for approval, the following treatment-related adverse events occurred in 1% or more of the 656 subjects with non-intact tympanic membranes in AOM TT or CSOM treated twice-daily with ofloxacin otic solution:

- Other treatment-related adverse reactions reported in subjects with non-intact tympanic membranes included: diarrhea (0.6%), nausea (0.3%), vomiting (0.3%), dry mouth (0.5%), headache (0.3%), vertigo (0.5%), otorrhagia (0.6%), tinnitus (0.3%), fever (0.3%). The following treatment-related adverse events were each reported in a single subject: application site reaction, otitis externa, urticaria, abdominal pain, dysaesthesia, hyperkinesia, halitosis, inflammation, pain, insomnia, coughing, pharyngitis, rhinitis, sinusitis, and tachycardia.
Postmarketing Experience
- Cases of uncommon transient neurospsychiatric disturbances have been included in spontaneous post-marketing reports. A causal relationship with ofloxacin otic solution 0.3% is unknown.
Drug Interactions
- Specific drug interaction studies have not been conducted with ofloxacin otic solution.
Use in Specific Populations
Pregnancy
Teratogenic Effects
- Ofloxacin has been shown to have an embryocidal effect in rats at a dose of 810 mg/kg/day and in rabbits at 160 mg/kg/day.
- These dosages resulted in decreased fetal body weights and increased fetal mortality in rats and rabbits, respectively. Minor fetal skeletal variations were reported in rats receiving doses of 810 mg/kg/day. Ofloxacin has not been shown to be teratogenic at doses as high as 810 mg/kg/day and 160 mg/kg/day when administered to pregnant rats and rabbits, respectively.
- Ofloxacin has not been shown to have any adverse effects on the developing embryo or fetus at doses relevant to the amount of ofloxacin that will be delivered ototopically at the recommended clinical doses.
Nonteratogenic Effects
- Additional studies in the rat demonstrated that doses up to 360 mg/kg/day during late gestation had no adverse effects on late fetal development, labor, delivery, lactation, neonatal viability, or growth of the newborn. There are, however, no adequate and well-controlled studies in pregnant women. Ofloxacin otic solution should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ofloxacin (otic) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Ofloxacin (otic) during labor and delivery.
Nursing Mothers
- In nursing women, a single 200 mg oral dose resulted in concentrations of ofloxacin in milk which were similar to those found in plasma. It is not known whether ofloxacin is excreted in human milk following topical otic administration. Because of the potential for serious adverse reactions from ofloxacin in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- Safety and efficacy have been demonstrated in pediatric patients of the following ages for the listed indications:
- Six months and older: otitis externa with intact tympanic membranes
- One year and older: acute otitis media with tympanostomy tubes
- Twelve years and older: chronic suppurative otitis media with perforated tympanic membranes
- Safety and efficacy in pediatric patients below these ages have not been established.
- Although no data are available on patients less than age 6 months, there are no known safety concerns or differences in the disease process in this population that will preclude use of this product.
- No changes in hearing function occurred in 30 pediatric subjects treated with ofloxacin otic and tested for audiometric parameters. Although quinolones, including ofloxacin, have been shown to cause arthropathy in immature animals after systemic administration, young growing guinea pigs dosed in the middle ear with 0.3% ofloxacin otic solution for one month showed no systemic effects, quinolone-induced lesions, erosions of the cartilage in weight-bearing joints, or other signs of arthropathy.
Geriatic Use
There is no FDA guidance on the use of Ofloxacin (otic) in geriatric settings.
Gender
There is no FDA guidance on the use of Ofloxacin (otic) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Ofloxacin (otic) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Ofloxacin (otic) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Ofloxacin (otic) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Ofloxacin (otic) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Ofloxacin (otic) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Otic
Monitoring
There is limited information regarding Ofloxacin (otic) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Ofloxacin (otic) and IV administrations.
Overdosage
There is limited information regarding Ofloxacin (otic) overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Mechanism of Action
- Ofloxacin is a broad-spectrum antibiotic that is active against both Gram-positive and Gram-negative bacteria. It functions by inhibiting DNA gyrase, a type II topoisomerase, and topoisomerase IV, which is an enzyme necessary to separate (mostly in prokaryotes, in bacteria in particular) replicated DNA, thereby inhibiting bacterial cell division.
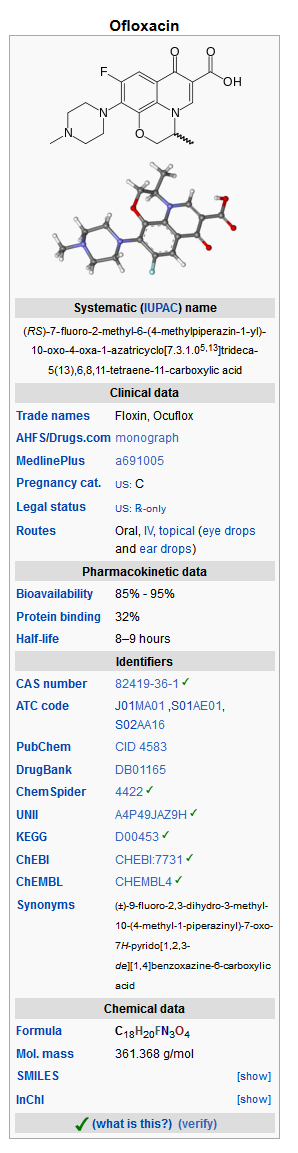
Structure
- Ofloxacin Otic Solution 0.3% is a sterile aqueous anti-infective (anti-bacterial) solution for otic use. Chemically, ofloxacin has three condensed 6-membered rings made up of a fluorinated carboxy-quinolone with a benzoxazine ring. The chemical name of ofloxacin is: (±)-9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid. The empirical formula of ofloxacin is C18H20FN3O4 and its molecular weight is 361.38. The structural formula is:

- Ofloxacin Otic Solution contains 0.3% (3 mg/mL) ofloxacin with benzalkonium chloride (0.0025%), hydrochloric acid, sodium chloride, and water for injection. Additional hydrochloric acid and/or sodium hydroxide may be added to adjust the pH (6.5 ± 0.5).
Pharmacodynamics
Microbiology
- Ofloxacin has in vitro activity against a wide range of gram-negative and gram-positive microorganisms. Ofloxacin exerts its antibacterial activity by inhibiting DNA gyrase, a bacterial topoisomerase. DNA gyrase is an essential enzyme which controls DNA topology and assists in DNA replication, repair, deactivation, and transcription. Cross-resistance has been observed between ofloxacin and other fluoroquinolones. There is generally no cross-resistance between ofloxacin and other classes of antibacterial agents such as beta-lactams or aminoglycosides.
- Ofloxacin has been shown to be active against most isolates of the following microorganisms, both in vitro and clinically in otic infections as described in the INDICATIONS AND USAGE section.
Aerobic and facultative gram-positive microorganisms:
- Staphylococcus aureus
- Streptococcus pneumoniae
- Aerobic and facultative gram-negative microorganisms:
- Escherichia coli
- Haemophilus influenzae
- Moraxella catarrhalis
- Proteus mirabilis
- Pseudomonas aeruginosa
Pharmacokinetics
- Drug concentrations in serum (in subjects with tympanostomy tubes and perforated tympanic membranes), in otorrhea, and in mucosa of the middle ear (in subjects with perforated tympanic membranes) were determined following otic administration of ofloxacin solution. In two single-dose studies, mean ofloxacin serum concentrations were low in adult patients with tympanostomy tubes, with and without otorrhea, after otic administration of a 0.3% solution (4.1 ng/mL (n=3) and 5.4 ng/mL (n=5), respectively). In adults with perforated tympanic membranes, the maximum serum drug level of ofloxacin detected was 10 ng/mL after administration of a 0.3% solution. Ofloxacin was detectable in the middle ear mucosa of some adult subjects with perforated tympanic membranes (11 of 16 subjects). The variability of ofloxacin concentration in middle ear mucosa was high. The concentrations ranged from 1.2 to 602 μg/g after otic administration of a 0.3% solution. Ofloxacin was present in high concentrations in otorrhea (389 - 2850 μg/g, n=13) 30 minutes after otic administration of a 0.3% solution in subjects with chronic suppurative otitis media and perforated tympanic membranes. However, the measurement of ofloxacin in the otorrhea does not necessarily reflect the exposure of the middle ear to ofloxacin.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Long-term studies to determine the carcinogenic potential of ofloxacin have not been conducted. Ofloxacin was not mutagenic in the Ames test, the sister chromatid exchange assay (Chinese hamster and human cell lines), the unscheduled DNA synthesis (UDS) assay using human fibroblasts, the dominant lethal assay, or the mouse micronucleus assay. Ofloxacin was positive in the rat hepatocyte UDS assay, and in the mouse lymphoma assay. In rats, ofloxacin did not affect male or female reproductive performance at oral doses up to 360 mg/kg/day. This would be over 1000 times the maximum recommended clinical dose, based upon body surface area, assuming total absorption of ofloxacin from the ear of a patient treated with ofloxacin otic solution twice per day.
Clinical Studies
There is limited information regarding Ofloxacin (otic) Clinical Studies in the drug label.
How Supplied
Ofloxacin Otic Solution 0.3% is supplied in bottles containing 5 mL.
5 mL bottle - 68788-9504-5
247807 January 2007

Storage
- Store at 20° to 25°C (68° to 77°F).
Images
Drug Images
{{#ask: Page Name::Ofloxacin (otic) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
Ofloxacin Otic Solution 0.3%
Rx

{{#ask: Label Page::Ofloxacin (otic) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Avoid contaminating the applicator tip with material from the fingers or other sources. This precaution is necessary if the sterility of the drops is to be preserved. Systemic quinolones, including ofloxacin, have been associated with hypersensitivity reactions, even following a single dose. Discontinue use immediately and contact your physician at the first sign of a rash or allergic reaction.
Otitis Externa
- Prior to administration of ofloxacin otic solution, the solution should be warmed by holding the bottle in the hand for one or two minutes to avoid dizziness which may result from the instillation of a cold solution. The patient should lie with the affected ear upward, and then the drops should be instilled. This position should be maintained for five minutes to facilitate penetration of the drops into the ear canal. Repeat, if necessary, for the opposite ear.
Acute Otitis Media and Chronic Suppurative Otitis Media
- Prior to administration of ofloxacin otic solution, the solution should be warmed by holding the bottle in the hand for one or two minutes to avoid dizziness which may result from the instillation of a cold solution. The patient should lie with the affected ear upward, and then the drops should be instilled. The tragus should then be pumped 4 times by pushing inward to facilitate penetration of the drops into the middle ear. This position should be maintained for five minutes. Repeat, if necessary, for the opposite ear.





Precautions with Alcohol
Alcohol-Ofloxacin (otic) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Floxin
- Ocuflox
Look-Alike Drug Names
There is limited information regarding Ofloxacin (otic) Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.