Odevixibat
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Tejasvi Aryaputra
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Odevixibat is a ileal bile acid transporter inhibitor that is FDA approved for the treatment of pruritus. Common adverse reactions include abdominal pain,, fat-soluble vitamin deficiency, diarrhea, vomiting, and liver test abnormalities..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Recommended Dosage
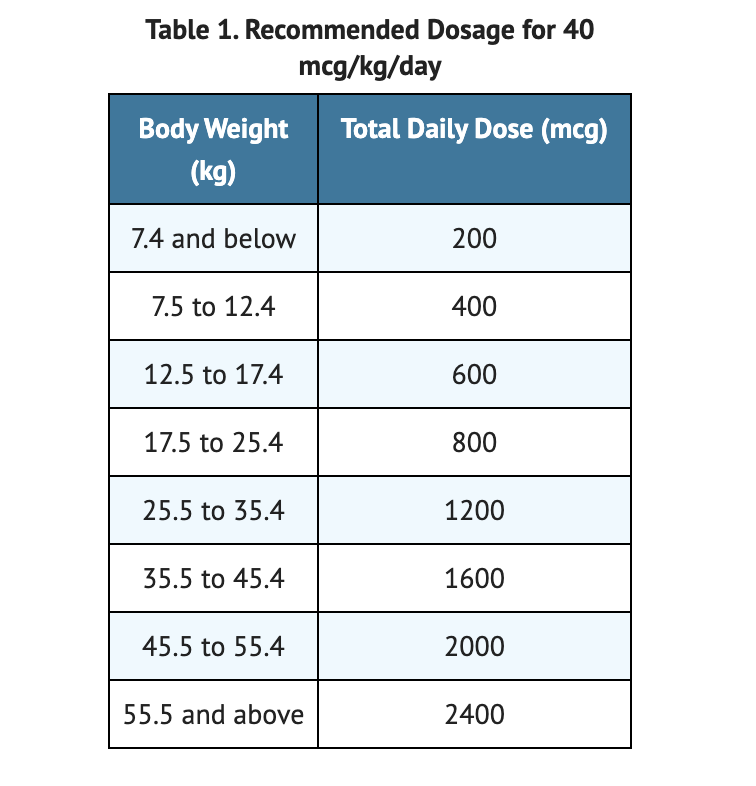
- 40 mcg/kg once daily of Odevixibat is the recommended dosage.
- Dosage should be taken with a meal in the morning.
- Recommended dosage may be increased to 120 mcg/kg if there are no signs of pruritus improvement after 3 months of Odevixibat treatment.
- Patients with a weight less than 19.5 kilograms may use Odevixibat pellets.
- Patients with a weight of 19.5 kilograms or more may use Odevixibat capsules.
Table 1 shows Recommended Dosage for 40 mcg/kg/day.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Odevixibat in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Odevixibat in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Odevixibat FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Odevixibat in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Odevixibat in pediatric patients.
Contraindications
There are no contraindications associated with Odevixibat.
Warnings
Liver Test Abnormalities
- Trial 1 clinical studies show elevations of liver tests or worsening of liver tests in comparison to baseline values for patients taking Odevixibat.
- Elevations of ALT, AST, or total and direct bilirubin were some of the abnormalities seen in the clinical studies.
- 3-120 days was the range of Odevixibat treatment interruption that occurred during the Trial 1 studies.
- Advise patients that treatment discontinuation of Odevixibat may be necessary if they experience persistent or recurrent liver test abnormalities.
Table 2 shows the Liver Test Data obtained in Trial 1 Clinical Studies.

Diarrhea
- 21% of patients receiving 120 mcg/kg/day of Odevixibat experienced diarrhea in Trial 1 clinical studies.
- 39% of patients receiving 40 mcg/kg/day of Odevixibat experienced diarrhea in Trial 1 clinical studies.
- 10% of placebo-treated patients experienced diarrhea in Trial 1 clinical studies.
- Treatment interruption was seen in patients receiving 120 mcg/kg/day of Odevixibat in Trial 1 clinical studies.
- Monitor patients for dehydration if they experience diarrhea and treat accordingly.
- Advise patients that interruption of Odevixibat treatment may be necessary if diarrhea persists.
Fat-Soluble Vitamin (FSV) Deficiency
- Advise patients that absorption of fat-soluble vitamins may occur during Odevixibat treatment.
- No patients receiving 40 mcg/kg/day of Odevixibat experienced new onset or worsening of existing FSV deficiency in Trial 1 clinical studies.
- 16% of patients receiving 120 mcg/kg/day of Odevixibat experienced new onset or worsening of existing FSV deficiency in Trial 1 clinical studies.
- 5% of placebo-treated patients experienced new onset or worsening of existing FSV deficiency in Trial 1 clinical studies.
- Monitor patients serum FSV levels at baseline and during Odevixibat treatment.
Adverse Reactions
Clinical Trials Experience
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions and durations of follow up, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- Trial 1 clinical study is a randomized, double-blind, placebo-controlled, 24-week study that looked into the effects of administering 40 mcg/kg vs 120 mcg/kg of Odevixibat. The study consisted of 62 patients that either received a placebo (20), 40 mcg/kg of Odevixibat (23), or 120 mcg/kg of Odevixibat (19).
- 85% of patients experienced AEs in Trial 1 Study.
- Abdominal pain, liver test abnormalities, fat-soluble vitamin deficiency, diarrhea, and vomiting were the most common adverse reactions reported by patients in Trial 1 clinical studies.
Table 3 shows the Clinical Adverse Reactions reported by patients in Trial 1 clinical studies.

- Trial 2 is a 72-week, open-label, single-arm trial that looked into the effects of Odevixibat in PFIC type 1, 2, and 3 patients. 79 patients were part of this trial with an age that ranged from 4 months to 25 years. Patients received 120 mcg/kg/day of Odevixibat once daily.
- Abdominal pain, liver test abnormalities, fat-soluble vitamin deficiency, diarrhea, and vomiting were the most common adverse reactions reported by patients in Trial 2 clinical studies.
- Liver test abnormalities led to Odevixibat treatment interruptions.
- 12 patients had to discontinue Odevixibat treatment in Trial 2 studies.
Postmarketing Experience
There is limited information regarding Odevixibat Postmarketing Experience in the drug label.
Drug Interactions
Bile Acid Binding Resins
- When taking Odevixibat, administration of bile acid binding resins at least 4 hours before or 4 hours after administration of Odevixibat.
- Odevixibat efficacy may be reduced if the bile acid binding resins binds with Odevixibat in the gut.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no data done on pregnant women treated with Odevixibat to determine the effects of Odevixibat on miscarriage, fetal outcomes, major birth defects, and adverse maternal outcomes. Cardiac malformations may occur in the fetus during pregnancy based on animal studies. Pregnant rabbit studies show how Odevixibat can increase malformations in fetal heart, great blood vessels, and other vascular sites in the fetus. During the period of organogenesis, fetus in pregnant rabbits experienced malformations of 5-chambered heart, small ventricle, large atrium, ventricular septum defect, misshapen aortic valve, dilated aortic arch, right sided and retroesophageal aortic arch, fusion of aortic arch and pulmonary trunk, ductus arteriosus atresia, and absence of subclavian artery when given 2.1 times the maximum recommended dose of Odevixibat. Increase in skeletal variations was seen in pregnant rat studies testing the effects of Odevixibat on the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Odevixibat in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Odevixibat during labor and delivery.
Nursing Mothers
Nursing should not cause exposure of Odevixibat to infants. No data is present on the effects done on the breastfed child and the effects on milk production when treated with Odevixibat. Absorption of fat-soluble vitamins may occur when taking Odevixibat. Monitor patients and the breastfed infant fat-soluble vitamins levels during nursing.
Pediatric Use
Studies done on pediatric patients 3 months to 17 years of age for the treatment of pruritus in PFIC looked into the safety and effectiveness of Odevixibat. Trial studies show that patients treated with Odevixibat had greater improvement in pruritus than patients receiving the placebo. Gastrointestinal symptoms, liver test abnormalities, and fat-soluble vitamin deficiency were the most common adverse reactions reported in Trial 1 and 2 patients. There are no studies done on pediatric patients less than 3 months of age that test the safety and effectiveness of Odevixibat.
Geriatic Use
Studies have not been conducted on PFIC in adult patients that look at the safety and effectiveness of Odevixibat.
Gender
There is no FDA guidance on the use of Odevixibat with respect to specific gender populations.
Race
There is no FDA guidance on the use of Odevixibat with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Odevixibat in patients with renal impairment.
Hepatic Impairment
Studies have not been conducted on PFIC patients with clinically significant portal hypertension and in patients with decompensated cirrhosis to look at the safety and effectiveness of Odevixibat. Hepatic function may be impaired in patients with PFIC at baseline.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Odevixibat in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Odevixibat in patients who are immunocompromised.
Administration and Monitoring
Administration
Preparation and Administration Instructions
- Take Odevixibat at least 4 hours before or 4 hours after taking a bile acid binding resin.
- Advise patients that capsules should not be crushed or chewed.
Oral Pellet Administration
- Odevixibat should be taken during morning meal.
- Contents of oral pellet should be mixed in soft food by gently tapping oral pellet.
- Administer dosage immediately after gently mixing contents of the pellet.
- Advise patients that shell containing oral pellets should not be swallowed whole.
- Odevixibat should not be taken by patients who are exclusively on liquid food.
Capsule Administration
- Advise patients that Odevixibat should be taken in the morning with a meal.
- Advise patients to swallow capsule whole with water.
Monitoring
Dose Modification for Management of Adverse Events
- Monitor patients Liver tests and watch for new onset liver test abnormalities during Odevixibat treatment.
- Liver tests include looking at AST, ALT, Total Bilirubin, Direct Bilirubin, and International Normalized Ratio.
- Advise patients that dosage interruptions may occur if patient experiences symptoms consistent with clinical hepatitis or new onset liver test abnormalities.
- Advise patients that the discontinuation of Odevixibat should occur if patient experiences a hepatic decompensation event.
IV Compatibility
There is limited information regarding the compatibility of Odevixibat and IV administrations.
Overdosage
There is limited information regarding Odevixibat overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
| |
Odevixibat
| |
| Systematic (IUPAC) name | |
| (2S)-2-{[(2R)-2-[({[3,3-Dibutyl-7-(methylsulfanyl)-1,1-dioxido-5-phenyl-2,3,4,5-tetrahydro-1,2,5-benzothiadiazepin-8-yl]oxy}acetyl)amino]-2-(4-hydroxyphenyl)acetyl]amino}butanoic acid | |
| Identifiers | |
| CAS number | |
| ATC code | A05 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | ? |
| Synonyms | A4250 |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | By mouth |
Mechanism of Action
- Odevixibat is a reversible inhibitor of the ileal bile acid transporter.
- The role of Odevixibat in the terminal ileum is to decrease the reabsorption of bile acids.
- Inhibition of IBAT may be the mechanism of Odevixibat that may improve pruritus in PFIC patients.
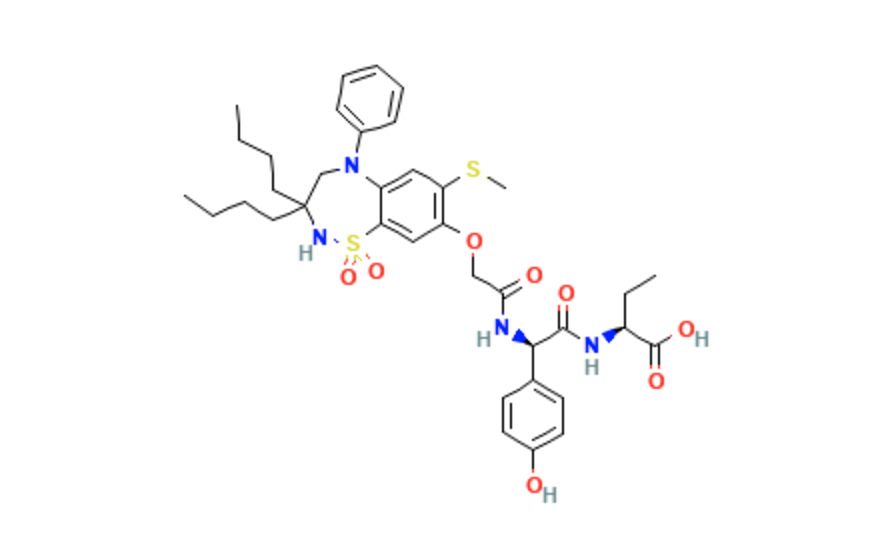
Structure
- Odevixibat is a reversible inhibitor of the ileal bile acid transporter. It has an empirical formula of C37H48N4O8S2.
- The molecular weight is 740.9 g/mol.

Pharmacodynamics
- PFIC patients experience a reduction of serum bile acids when taking Odevixibat.
- 88.7% of patients in Trial 1 clinical studies had elevated serum bile acids above 100 µmol/L at baseline which eventually decreased in 4-8 weeks of Odevixibat treatment.
- Serum bile acids reduction were similar in patients receiving either 40 or 120 mcg/kg of Odevixibat.
Pharmacokinetics
- 0.06 to 0.72 ng/mL was the range of measurable Odevixibat concentrations in pediatric patients with PFIC receiving 40 mcg/kg or 120 mcg/kg once daily of Odevixibat.
- Plasma concentrations were below the limit of quantification in healthy adults receiving 0.1 to 3 mg of Odevixibat.
- 0.47 ng/mL is the mean Cmax in patients who were given a single administration of 7.2 mg of Odevixibat.
- 2.19 ng*h/mL is the AUC0-24h in patients who were given a single administration of 7.2 mg of Odevixibat.
Absorption
- 1 to 5 hours is when Cmax is reached in healthy adults receiving 7.2 mg of Odevixibat.
- Sprinkling of apple sauce on pellets caused a 39% decrease in Cmax when patients were given 9.6 mg of Odevixibat.
- Sprinkling of apple sauce on pellets caused a 35% decrease in AUC0-24h when patients were given 9.6 mg of Odevixibat.
- Sprinkling of apple sauce on pellets caused a 3 hours to 4.5 hours delay in median Tmax when patients were given 9.6 mg of Odevixibat.
Effect of Food
- 3 hours to 4.5 hours is the range of the delay of median Tmax with the co-administration of 9.6 mg of Odevixibat with a high-fat meal.
- There is a 72% reduction in Cmax with the co-administration of 9.6 mg of Odevixibat with a high-fat meal.
- There is a 62% reduction in AUC0-24h with the co-administration of 9.6 mg of Odevixibat with a high-fat meal.
Distribution
- In vitro, the human plasma protein binding is greater than 99% for Odevixibat.
Elimination
- 2.36 hours is the mean half-life in patients who were given 7.2 mg Odevixibat.
Metabolism
- Mono-hydroxylation metabolizes Odevixibat in vitro.
Excretion
- 82.9% of Odevixibat was recovered in feces in which 97% was found unchanged after patients were given a single radiolabeled Odevixibat 3 mg oral dose.
- Less than 0.002% of Odevixibat was recovered in urine after patients were given a single radiolabeled Odevixibat 3 mg oral dose.
Drug Interaction Studies
Effect of Other Drugs on Odevixibat:
- Odevixibat is a substrate of P-glycoprotein.
- Odevixibat is not a substrate of breast cancer resistance protein.
- 66% increase of AUC0-24h was seen with the co-administration of 7.2 mg of Odevixibat and itraconazole.
- 52% increase of Cmax was seen with the co-administration of 7.2 mg of Odevixibat and itraconazole.
Effect of Odevixibat on Other Drugs:
- CYP isoforms 2B6, 2C8, 2C9, 2D6, 1A2, or 2C19 are not inhibited by Odevixibat in vitro studies.
- CYP isoforms 1A2, 2B6, or 3A4 are not induced by Odevixibat in vitro studies.
- 29% decrease in AUC0-24h of Midazolam was seen with the co-administration of 7.2 mg of Odevixibat and oral midazolam.
- 13% decrease in 1-OH midazolam was seen with the co-administration of 7.2 mg of Odevixibat and oral midazolam.
- Transporters P-gp; BCRP; organic anion transporter polypeptide 1B1 and 1B3(OATP1B1 and OATP1B3); organic anion transporter (OAT)1, OAT3; organic cation transporter 2 (OCT2), multidrug and toxin extrusion transporter 1 and 2K (MATE1 and MATE2K) are not inhibited by Odevixibat in vitro.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis:
- When rats or mice were given doses up to 100 mg/kg/day, Odevixibat was not tumorigenic.
- 231 and 459 times is the systemic exposure to Odevixibat in rats and mice.
Mutagenesis:
- Odevixibat was negative in the vitro mouse lymphoma cell gene mutation assay, vitro bacterial reverse mutation (Ames) assay, and in vivo rat micronucleus test.
Impairment of Fertility
- Male and female rats did not experience any effects on fertility or reproductive function when given Odevixibat oral doses of up to 1000 mg/kg/day.
Clinical Studies
Trial 1
- Trial 1 is a 24-week, randomized, double-blind, placebo-controlled trial.
- 62 pediatric patients with PFIC type 1 or type 2 were part of the Trial 1 study.
- Patients were either part of the placebo group, 40 mcg/kg of Odevixibat group, or 120 mcg/kg of Odevixibat group.
- The patient population was largely Caucasian (84%), included 50% of males, and had a mean age of 3.2 years of age.
- 19% of patients in the Odevixibat groups had to discontinue treatment due to adverse reactions or no improvement in pruritus was seen from Trial 1 clinical studies.
- 25% of patients in the placebo group had to discontinue treatment due to adverse reactions or no improvement in pruritus was seen from Trial 1 clinical studies.
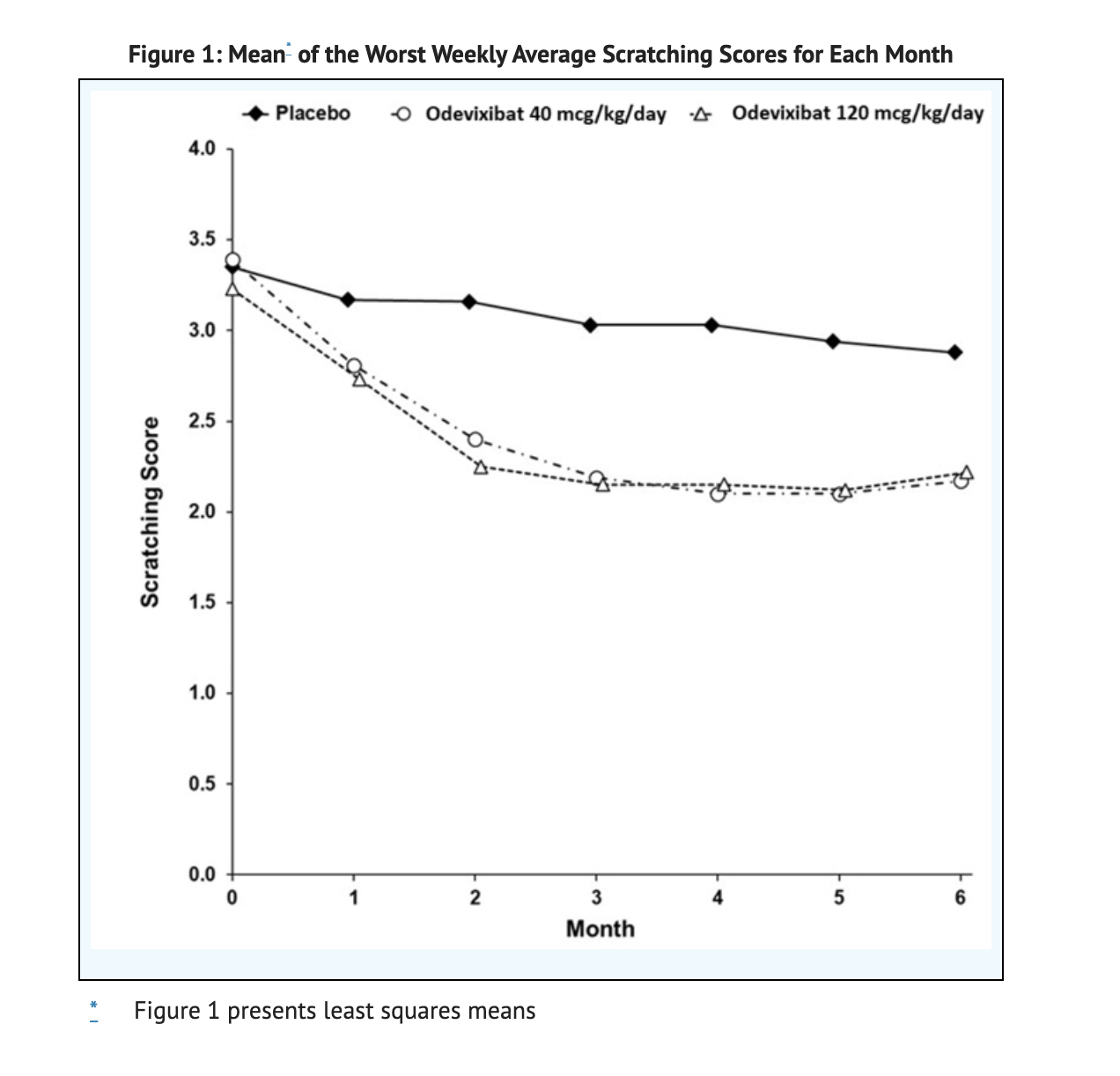
- Patients scratching was measured using a single-item observer-reported outcome during the Trial 1 studies.
- Scratching was measured on a 5 point scale where 0 is no scratching to 4 which is the worst possible scratching.
Table 4 displays Efficacy Results Over the 24-Week Treatment Period in Patients with PFIC Type 1 or 2 in Trial 1.
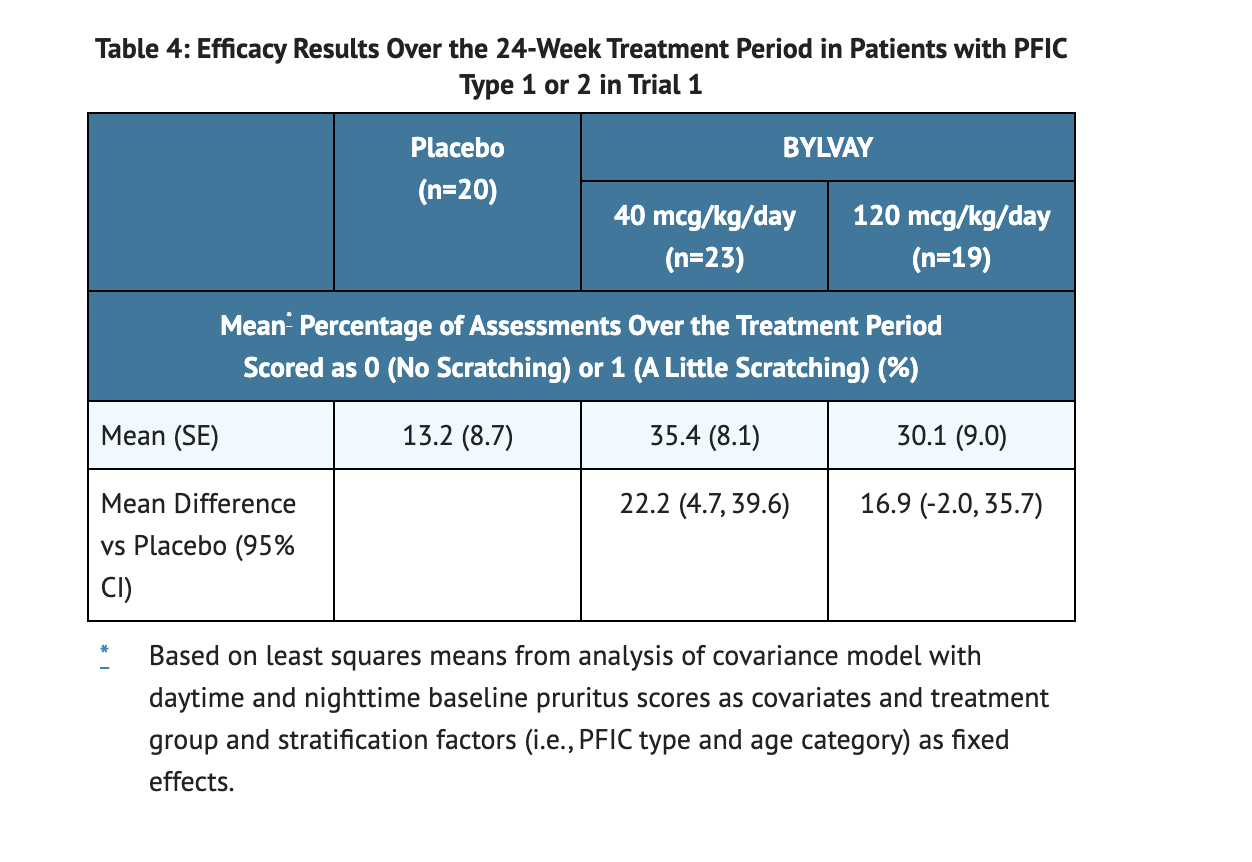
Figure 1 shows the Mean of the Worst Weekly Average Scratching Scores for Each Month.
How Supplied
Oral Pellets
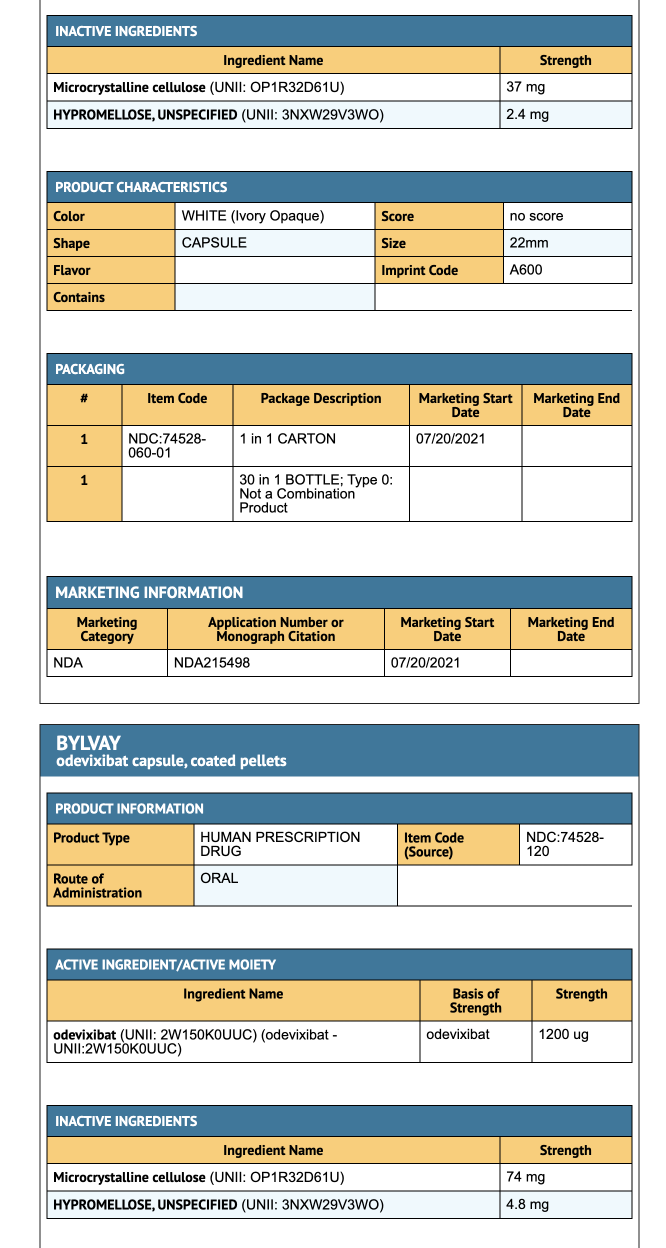
- 200 mcg Oral Pellets: Ivory opaque cap and white opaque bod.
- 600 mcg Oral Pellets: Ivory opaque cap and body
Capsules
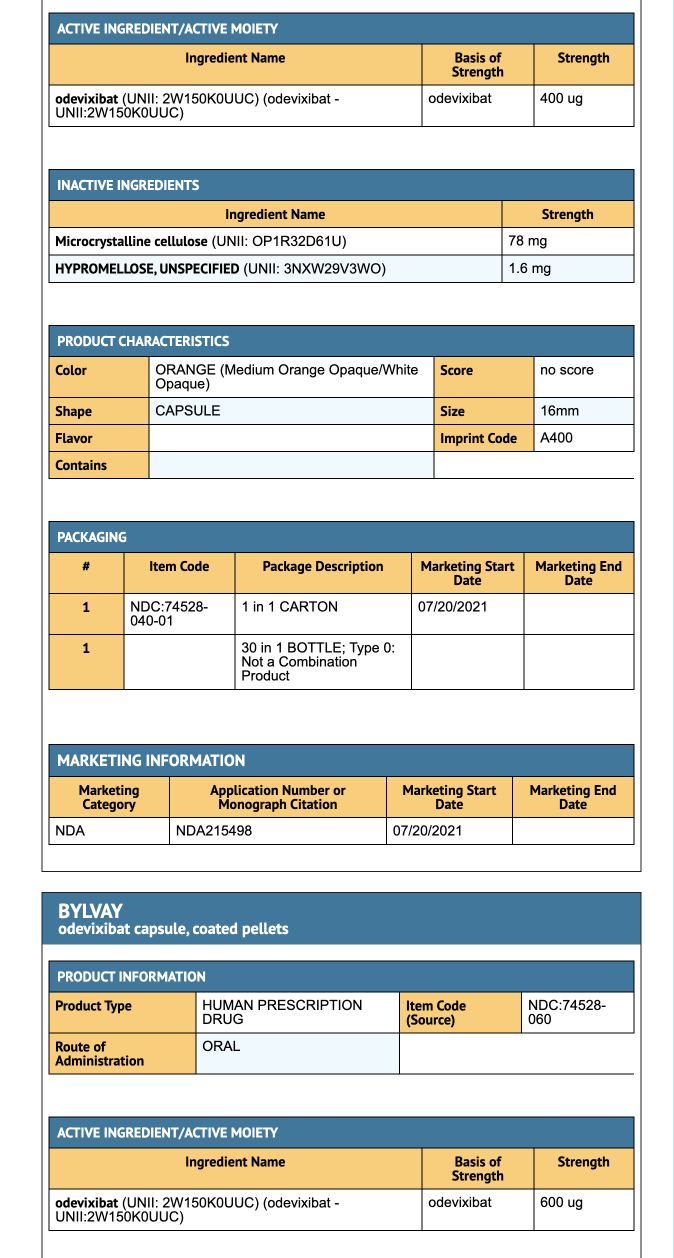
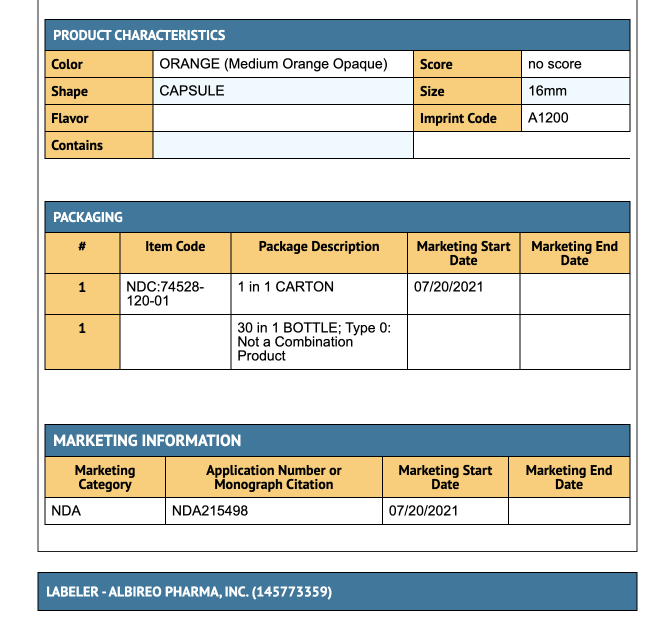
- 400 mcg Capsule: Medium orange opaque cap and white opaque body.
- 1200 mcg Capsule: Medium orange opaque cap and body.
Storage
- Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Images
Drug Images
{{#ask: Page Name::Odevixibat |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Odevixibat |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Risks
- Advise patients that symptoms associated with Odevixibat treatment include vomiting, dehydration, abdominal pain, and diarrhea.
- Advise patients that elevations of liver tests may be a result of Odevixibat treatment.
- Monitor patients liver test results before and during Odevixibat treatment.
- Advise patients to report any signs of liver problems and worsening diarrhea to their healthcare provider.
- Advise patients that the absorption of fat-soluble vitamins during Odevixibat treatment.
Administration
- Advise patients that Odevixibat should not be mixed with liquids.
- Advise patients that the 200 mcg or 600 mcg capsule pellets should not be swallowed whole.
- Advise patients who are taking bile acid binding resins to wait at least 4 hours before or 4 hours after taking a bile acid binding resin to take Odevixibat.
Precautions with Alcohol
Alcohol-Odevixibat interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Bylvay
Look-Alike Drug Names
There is limited information regarding Odevixibat Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.